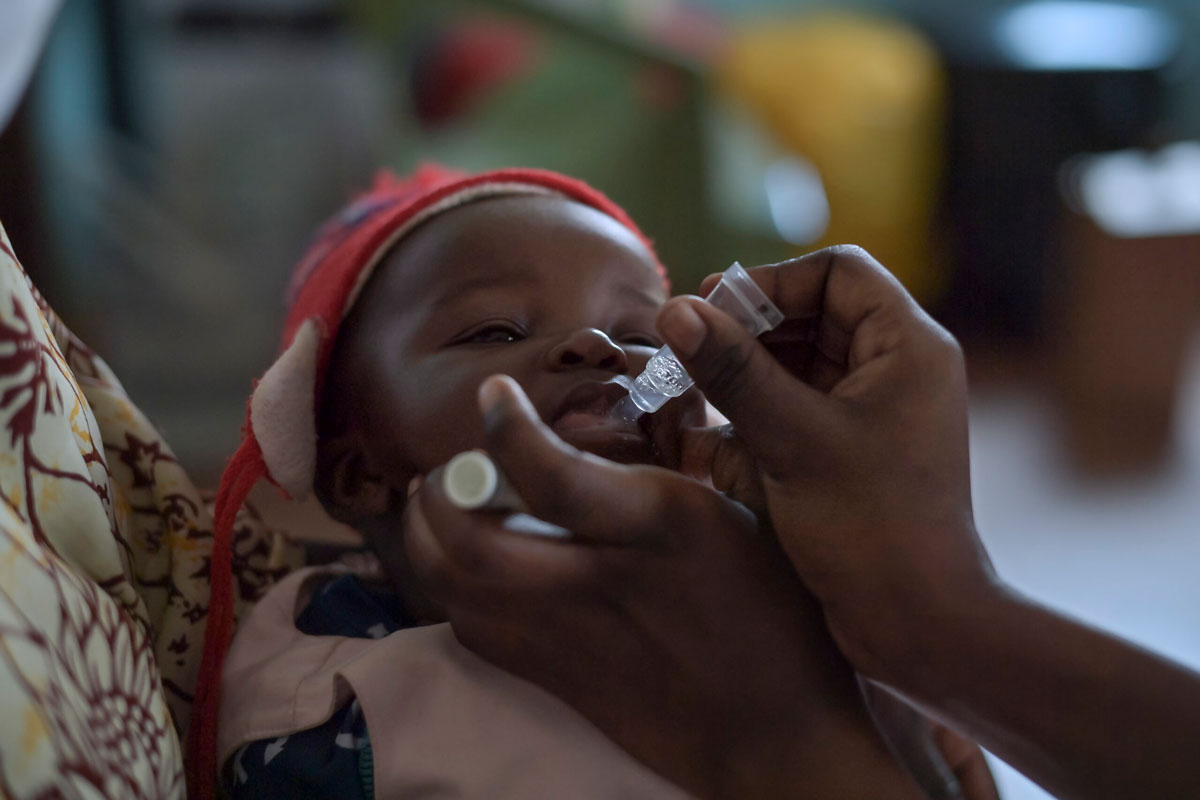
How combining vaccines saves money and lives
New vaccines are urgently needed, but fitting them into increasingly crowded immunisation schedules is challenging. Could combination vaccines provide a solution?
- 20 March 2025
- 12 min read
- by Linda Geddes

Twenty-five years ago, despite the existence of an effective vaccine, Haemophilus influenzae type b (Hib) was one of the biggest killers of young children and the main cause of meningitis worldwide.
When Gavi, the Vaccine Alliance was established in 2000, it made immunisation against this killer a top priority, but poor awareness about Hib and concerns about the cost of vaccination meant that progress was slow.
“Four years later, still only 15 out of 75 countries we supported had introduced the vaccine,” says Gavi’s Head of Policy, Dr Marta Tufet Bayona.
A turning point came in 2005, when Gavi’s Board decided that it would only support immunisation against another deadly infection – hepatitis B – through a combination vaccine, such as the five-in-one pentavalentvaccine that combines protection against hepatitis B, Hib, diphtheria, tetanus and pertussis in a single shot.
“The Hib vaccine on its own was not enough to drive uptake, but when it was combined with hepatitis B, this was able to drive coverage for both diseases,” Bayona says.
Today, every Gavi-supported country provides Hib immunisation through this combined approach – averting an estimated 1.4 million deaths between 2011 and 2020 alone.

Other combination vaccines have had a similarly impressive impact, from the three-in-one measles, mumps and rubella (MMR) vaccine to pneumococcal conjugate vaccines (PCVs), which protect against up to 20 strains of pneumonia- and meningitis-causing bacteria.
“The history of immunisation is a history of combination vaccines,” says Dr Bill Hausdorff, Head of Public Health Value Propositions at PATH's Center for Vaccine Innovation and Access in Washington DC.
Increasingly though, experts are eyeing combination vaccines as a solution to another pressing problem: although new vaccines are urgently needed, the logistics of delivering them is increasingly hindered by their own success.
“Despite the technological beauty of all these potential and current vaccines, we’ve hit a crossroads where the ability to make new vaccines is well surpassing countries’ abilities to absorb them,” says Hausdorff. “They don’t have the money; they don’t have room in their immunisation schedules.”
Combining some of these new vaccines into existing shots might simplify their delivery. It could also potentially reduce their cost. “If you have a single vial, you have less equipment, fewer issues around delivery and disposal of needles. You’re also reducing the environmental impact of vaccination,” says Bayona.
Yet, despite these benefits, few new combination vaccines are in the pipeline. Why is this, and what can be done to support their development?
Combined protection
The idea of combining multiple vaccines into the same injection has a long history. Decades before the pentavalent vaccine was introduced, the diphtheria, tetanus and pertussis-containing (DTP) vaccine formed a cornerstone of the World Health Organization’s Expanded Programme on Immunization (EPI) when it was launched in 1974.
“Vaccines for diphtheria, tetanus, pertussis were all developed separately, but it wasn’t until you put them together that you went from giving nine shots to little babies to just three shots,” says Hausdorff.
Many combination vaccines are marriages of convenience, rather than the pathogens they target having much in common with one another.
For instance, the pentavalent vaccine protects against four unrelated species of bacteria, plus a virus, each affecting different tissues and body systems. The main thing these pathogens have in common is that they are most dangerous to infants and young children, which means preventative vaccines need to be given early.
“Usually, what has determined the combination of vaccines has been the schedule – so, putting together vaccines that are otherwise given at around the same time – provided there’s no interference between them,” says Prof Rudzani Muloiwa, Head of the Department of Paediatrics and Child Health at the University of Cape Town and Deputy Chair of South Africa’s National Advisory Group on Immunization.
Have you read?
Existing vaccines already protect against 27 life-threatening viruses, bacteria and parasites, saving an estimated 4–5 million lives worldwide each year. Several more are expected to become available within the next five years – including a vaccine against the diarrhoeal disease Shigella and a new tuberculosis vaccine for adults and adolescents.
Yet, the immunisation programmes that deliver vaccines are becoming increasingly saturated.
The World Health Organization (WHO) currently recommends that children receive ten routine immunisations during their first two years of life, requiring at least six visits to a vaccination centre. Adding further vaccines or vaccination appointments will be logistically challenging, not to mention financially prohibitive for some governments.
These logistical challenges can create knock-on problems for vaccine developers, who need to be confident that there will be a market for their products before committing to developing and testing them.
“Providing that assurance is becoming more and more difficult because the schedule is so congested, and because of the budget constraints for new vaccine introductions,” says Dr Birgitte Giersing, team lead for vaccine prioritisation and platforms at WHO.
This is particularly true of vaccines against pathogens that don’t kill vast numbers of people but nonetheless cause significant illness or disability.
For instance, even though a vaccine against Shigella – a major cause of moderate to severe diarrhoea worldwide – has a high probability of technical success and could significantly reduce growth stunting and long-term disability, it is uncertain whether countries would introduce a standalone vaccine against it, says Hausdorff. “Combined with something else, though, a Shigella vaccine becomes a more interesting prospect.”

Simplified schedule
Another issue is that, although existing combination vaccines have reduced the number of injections children receive during immunisation appointments, multiple shots are still often necessary, which some caregivers object to.
“If a mother has brought her baby to the clinic to have three shots, and the baby is very upset after the first shot, there may not be an opportunity to administer the other two, meaning the baby leaves without the full intended regimen,” Giersing says.
Even if the caregiver intends to return later, missed or delayed immunisation leaves infants susceptible to disease, and in many cases, the postponed appointment never happens because it is impractical or too expensive to return.
Combining multiple vaccines into the same injection could simplify matters. “From demand studies, we know that fewer needles and fewer trips to vaccination centres result in greater acceptance of vaccines, which translates into higher coverage and therefore a greater impact on health,” Bayona says.
It could also help to reduce the cost of immunisation. For instance, South Africa replaced the pentavalent vaccine with a six-in-one or hexavalent vaccine – which also protects against polio – in 2016.
“What made us choose it wasn’t the fact that it would mean fewer injections, or that it was a useful thing to have. It gave us a strategy for the polio endgame at a lower cost,” says Muloiwa.
“The combination vaccine itself is the single most expensive vaccine we have in our schedule, but by putting a number of vaccines together, it created a much easier logistical framework. This made the cost of roll-out much lower, because suddenly you didn’t have to have multiple vaccines sitting on the shelf needing different storage methods.
“We tend to think about the cost of vaccination as the cost of the doses, but for some vaccines the operational costs – the healthcare workers, the syringes, the logistics, the cold chain and the rest of it – are a hidden cost, which can be much higher.”
Syndromic vaccines
There could be further benefits from combining vaccines. An exciting prospect is the possibility of combination vaccines against single clinical syndromes, such as diarrhoea or ear infections.
In both conditions, various bacteria or viruses could be the root cause, but if there were a vaccine that protected against the main offenders, this could reduce unnecessary overuse of antibiotics – a major driver of antimicrobial resistance worldwide.
“When you go to see a doctor with your baby who's got an ear infection, the doctor doesn't usually know what’s causing it, so they’ll tend to give antibiotics on the presumption that it's a bacterial infection – even though it could be a virus, and antibiotics don’t work against viruses,” says Hausdorff.
However, if a combination vaccine were developed that protected against the major bacterial causes, this would not only reduce a child’s risk of ear infections, but if they did develop one, their doctor would know it was likely caused by a virus. In both situations, this could reduce prescribing of antibiotics.
Similarly, diarrhoeal disease in low-income countries tends to be caused by one of three bacteria: Shigella, enterotoxigenic Escherichia coli (ETEC), or Campylobacter.
“All of these are linked, not just to diarrhoea, but ultimately to growth stunting. Children who are repeatedly infected don’t grow as well, and it can affect their cognitive development,” says Hausdorff. “If you had a vaccine against those three bacteria, you could not only have a huge impact on antibiotic use, but it could have an impact on stunting.”
Communicating the value of such ‘syndromic vaccines’ to caregivers might also be easier than explaining the value of vaccines against these individual bacteria, says Hausdorff: “Being able to tell a parent that the vaccine they’re being offered is against the major causes of ear infection, is an easier sell than saying it’s against Haemophilus influenzae type b.”
Commercial challenges
But if combination vaccines are so useful why are so few currently being developed? One issue is that although creating new combinations is technically feasible, it is not straightforward.
“People sometimes naively think that you can just pour two things into the same vial, and you’re done, but creating a new combination vaccine is almost always a highly complex process,” says Dr Thomas Breuer, Head of Global Health and former Head of Vaccine Development at GSK. “It takes years of research to make sure that one antigen doesn’t interfere with another one, and so on.”
It is also difficult to predict which combinations will be most wanted or needed in the future. For instance, GSK is currently testing a vaccine against two types of Salmonella bacteria that cause typhoid and paratyphoid fevers.
In theory, the company could also investigate the technical feasibility of combining further Salmonellaeantigens to protect against other infections, including non-typhoidal salmonella – a leading cause of bloodstream infections in sub-Saharan Africa.
“The challenge is we have to make decisions five to ten years before a vaccine comes to the market,” says Breuer. “And we have no direction on whether such a vaccine will be used in the future and can be paid for.”
Breuer, for one, would like a clearer steer from health organisations and countries, on which combinations they would like to see developed: “We need more frequent discussion platforms earlier on, where future decision-makers, like WHO, Gavi and regulatory agencies sit at the table and help us to steer what to prioritise, or help us proactively to define the path to licensure, so we don’t go running off in the wrong direction.”
Giersing agrees on the need for stronger and earlier dialogue with countries and vaccine manufacturers about future combination vaccines. With funding from the Gates Foundation, WHO and PATH are embarking on a two-year project to develop a framework to evaluate the programmatic and technical feasibility, as well as the value of specific combinations.
This would be used to help inform global, regional and advisory committees about potential combinations that could be priorities for future product development. They plan to consult with a broad spectrum of stakeholders in the process – including immunisation programme managers, policy decision-makers, vaccine manufacturers and other entities such as Gavi.
“From WHO’s perspective, we want to start by understanding programmatically where are the needs and opportunities for novel combination vaccines,” says Giersing.
“Once we have that, the next series of questions would address the technical feasibility of combining some of the candidates that are in development and finally what the regulatory challenges to their clinical development and approval could be, as well as the potential commercial barriers that would need to be addressed.”
For instance, there may be intellectual property complexities impeding access to multiple vaccine components, as well as scientific or manufacturing challenges to combining and testing them.
Also needed, according to Muloiwa, are earlier discussions about how countries can continue to access standalone vaccines, if combination vaccines covering them were to be introduced.
In the past, some manufacturers have stopped producing standalone vaccines – or smaller combination vaccines – once more comprehensive combinations were introduced. “But if you can’t afford a six- or seven-antigen vaccine, and there are two or three priority antigens that you could afford – but can’t access – what do you do?” Muloiwa says.
Speeding up the approval process
A further challenge is that the policy, regulatory and communications frameworks for assessing and approving new vaccines have largely been designed with standalone single-pathogen vaccines in mind. “When you come in with something more complicated, they’re not really set up to handle it,” says Hausdorff.
In a recent policy paper, he and others – including Bayona and Giersing – argued for a rethink of how novel combination vaccines are identified and assessed, drawing on regulatory pathways that catalysed the development, licensure and availability of pneumococcal conjugate vaccines and the recently approved MenFive vaccine, which protects against the five leading causes of bacterial meningitis in sub-Saharan Africa.
This includes reaching a consensus on the minimum core components to be included in advance, and recognising that proving efficacy for each individual component is extremely difficult in the context of clinical trials, so surrogate indicators of efficacy may be needed for some of them – followed up by post-licensing studies to assess the real-world impact of these vaccines.
Regulatory authorities could also give more weight to the overall clinical benefit of combinations and to the practical advantages of minimising the number of vaccine administrations – such as improved and more equitable coverage – in licensing decisions.
“Importantly, there should be no difference in the standards for safety, but the regulatory community has to think about the value of the vaccine overall, and not just focus on the individual parts, because the value of combinations is more than the individual parts,” Hausdorff said.
Achieving all of this will not be easy, requiring a fundamental shift in regulatory, policy and financing perspectives that recognise the inherent value of combination vaccines over standalone ones. Yet, it is worth the effort, if we wish to address some of the biggest health challenges or our time, from antimicrobial resistance to climate change.
Vaccines remain one of the most effective public health tools in our armoury. Combining them could further strengthen their power.