Everything you need to know about candidate vaccines and treatments for Marburg disease
As Rwanda battles the deadly Ebola-like virus, fast-tracked clinical trials could stop Marburg from spreading, and save lives.
- 8 October 2024
- 5 min read
- by Linda Geddes
Health workers are scrambling to contain an outbreak of Marburg disease in Rwanda, with at least 49 people infected and 12 deaths as of 6 October 2024.
The World Health Organization (WHO) has assessed the risk posed by the current outbreak of the Ebola-like disease as very high at the national level, and the risk of spread to neighbouring countries, such as the Democratic Republic of the Congo (DRC), as high.
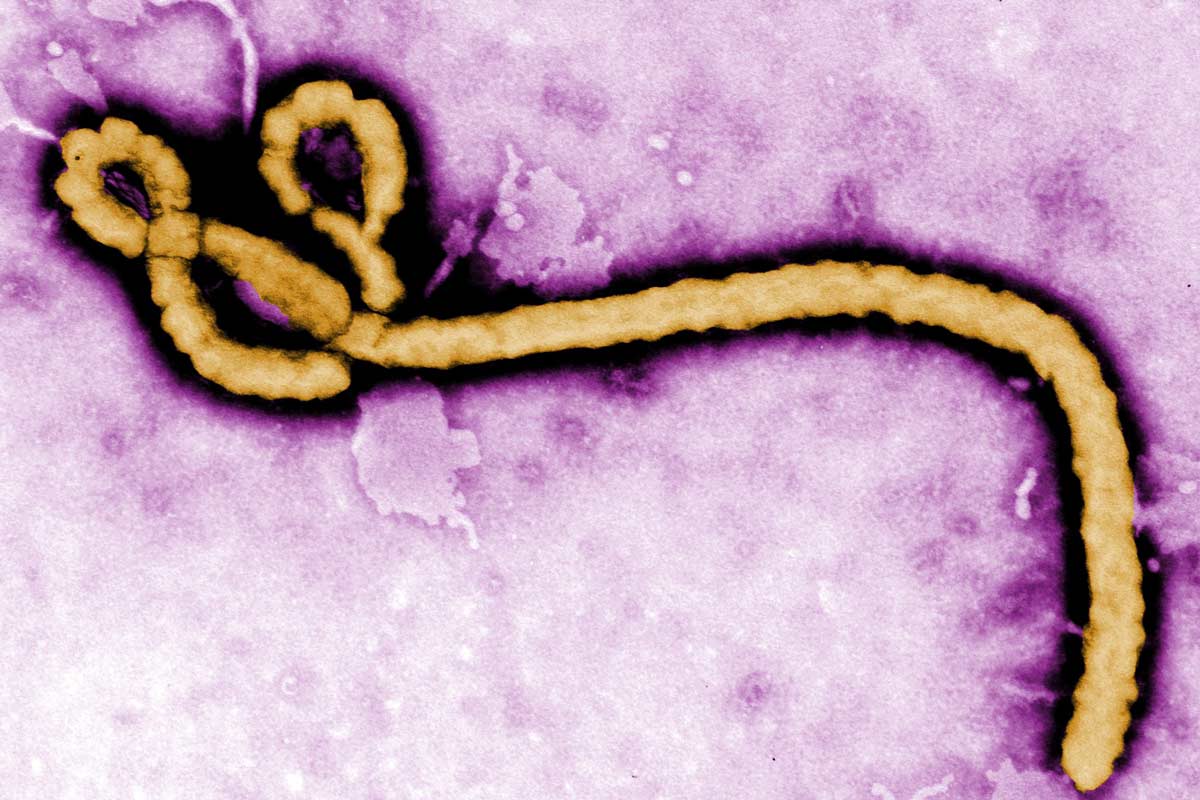
Like Ebola, Marburg causes fever, severe headaches, stomach pain, joint pain and diarrhoea. If the patient starts haemorrhaging, these flu-like symptoms can segue into vomiting blood, liver failure and neurological damage.
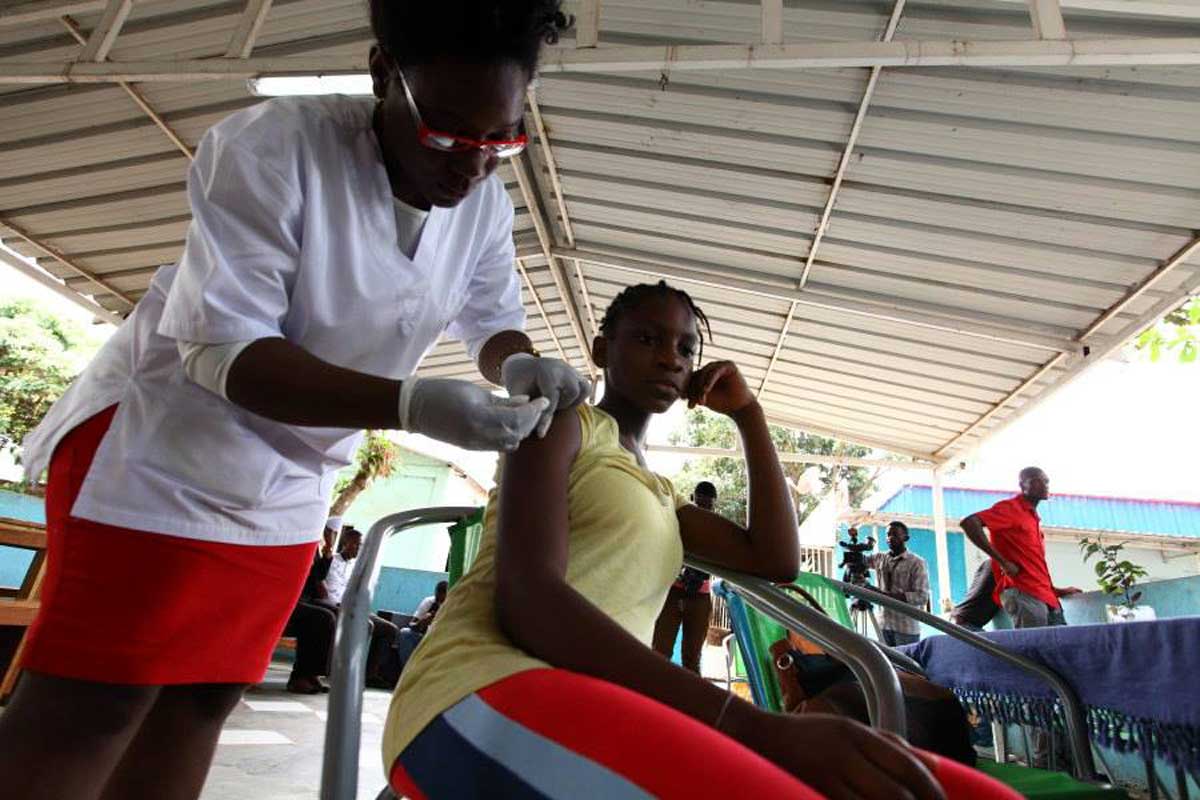
Although there are currently no licensed vaccines or treatments for Marburg disease, several candidates have shown promise in preclinical studies or early-stage clinical trials, and the urgency of the situation means that human trials are expected to begin within weeks, with shipments of experimental vaccines and therapeutics already beginning to arrive in Rwanda.
Scientists believe that the outbreak could help gather valuable data on the safety and effectiveness of these interventions, as well as helping to prevent further transmission of the virus, and potentially save lives. Here we look at the most advanced candidates and what we know about their efficacy so far.
Vaccine candidates
At least 28 vaccine candidates against Marburg virus are at various stages of development, and WHO has already prioritised four of them for evaluation in human trials, based on their safety, ability to provoke an immune response (immunogenicity), potential for efficacy, and availability for deployment in trials.
All of them are viral vector vaccines, which use a harmless, modified virus (the vector) to deliver genetic code for a Marburg virus protein into human cells. The cells then start manufacturing this viral protein, triggering a protective immune response against it.
All four candidates have been shown to protect against Marburg in animal models of the disease, but only one – an adenovirus-based vaccine developed by the Sabin Vaccine Institute in the US – has been fully evaluated in human trials so far. It is currently being tested in a phase 2 trial in 125 healthy adults in Uganda and Kenya, following a phase 1 trial that suggested it was safe, and elicited rapid and robust immune responses in 40 healthy US adults.
Approximately 700 doses of the Sabin Institute’s vaccine candidate are already being shipped to Rwanda for use in a phase 2 trial, which will initially involve approximately 700 high-risk adults, including health care workers.
The other Marburg vaccines that WHO has prioritised for testing include an additional adenovirus-based vaccine being developed at the University of Oxford, UK, which uses the same ChAdOx1 platform as Oxford’s COVID-19 vaccine. A phase 1 trial of this candidate began in the UK earlier this year.
The other two candidates, which are being developed by the International AIDS Vaccine Initiative (IAVI) and Public Health Vaccines LLC, use a different virus vector called vesicular stomatitis virus (VSV) to deliver the instructions for making a Marburg virus protein into human cells. The same vector underpins the Ervebo vaccine against Ebola, which WHO has prequalified for use in outbreaks caused by the Zaire ebolavirus.
Experimental drugs
WHO is also organising the SOLIDARITY PARTNERS trial, a clinical trial to find the best treatments for filoviruses – including Marburg and Ebola viruses. The first two agents it plans to evaluate in outbreak settings are the antiviral drug remdesivir – which is also used to treat COVID-19 – and a monoclonal antibody specific for Marburg virus disease known as MBP091.
Already, Gilead Sciences, which makes remdesivir, has announced that it will donate approximately 5,000 vials of the drug to Rwanda for emergency use in response to the outbreak. The drug works by inhibiting the replication of viruses, with preclinical studies suggesting it may be effective against various pathogens, including Marburg, Ebola, SARS and MERS. Other antiviral drugs, including galidesivir and favipiravir, have also shown promise against Marburg virus during preclinical studies – although none have yet proved their worth in human patients.
MBP091, meanwhile, is a monoclonal antibody, designed to replicate the antibody proteins that the body naturally produces to defend itself against pathogens. In this case, the monoclonal antibody is derived from a natural antibody that was isolated from a human patient who survived Marburg disease, and can now be mass-produced in a laboratory. Preclinical studies have suggested that it binds to Marburg virus and reduces its ability to infect human cells, although its efficacy against Marburg disease in human patients is currently unknown.
A spokesperson for the US Administration for Strategic Preparedness and Response – a division of the Department of Health and Human Services – told STAT magazine that it was supplying doses of MPB091 for use in Rwanda.
Details of precisely how these therapies will be used during the current outbreak are still unclear, but a previous study in macaques suggested that combining MPB091 with remdesivir could potentially extend the therapeutic window during which they helped to prevent deaths from Marburg disease.
Have you read?
Current outbreak
While studies of these drug and vaccine candidates are welcome, it is likely to be some time before we know if they are effective or not. For now, Rwanda’s Ministry of Health is urging people to be aware of the symptoms of Marburg disease to maintain good hygiene and avoid contact with symptomatic people, and to quickly call 114 if they develop symptoms themselves.