Speeding up vaccine development: Can we go from lab to jab in just 100 days?
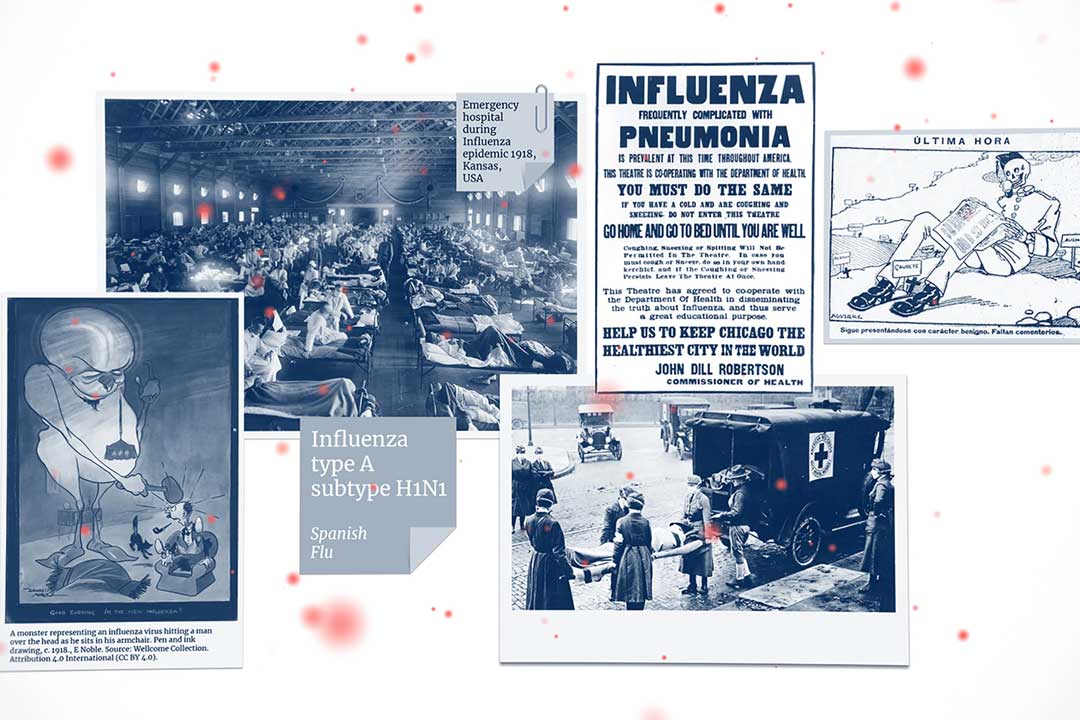
COVID-19 vaccines were made in record speed, taking around 300 days from the moment the threat was first identified. But the world’s top scientists are aiming to overtake this world record in the next pandemic, aiming to make a vaccine in 100 days.
- 8 June 2021
- 5 min read
- by CEPI

As soon as the COVID-19 virus was first identified, scientists around the world rapidly began work on COVID-19 vaccines, but few could have predicted that they would be ready for roll-out at in less than a year. The lightning speed at which they were produced – faster by a long margin than any vaccine development previously – left the scientific community, and the world, wondering how we can beat this record. Now, governments and science organisations across the globe are setting a goal that the next time there’s a pandemic threat, we will get a vaccine ready in 100 days.
The new US White House Science Advisor Eric Lander described this goal as “totally feasible” in his first interview after being sworn in to government. CEPI (the Coalition for Epidemic Preparedness Innovations) was at the forefront of funding and guiding COVID-19 vaccine development, and is a partner along with Gavi in the COVAX Facility that ensures equitable access to COVID-19 vaccines. The organisation's head of vaccine research and development Dr Melanie Saville also wants to see the next pandemic vaccine created in 100 days, and CEPI is looking into the technology needed to make that happen. The UK meanwhile has seen a major public-private collaboration on the ‘100 Days Mission’ to develop and deploy vaccines, diagnostics and therapeutics no more than 100 days after a pandemic threat has been identified.
How we made COVID-19 vaccines so fast
While vaccines against the new coronavirus might seem to have been developed at breakneck speed, they actually built on years of scientific progress.
SARS-CoV-2, the virus that causes COVID-19, was not the first coronavirus to threaten human health. Research into vaccines for two previous global threats from closely related coronaviruses, SARS and MERS-CoV, had led to a solid understanding that an effective COVID-19 vaccine would need to target the ‘spike protein’ on the surface of all three coronaviruses.
Radically reducing the speed of vaccine development will rely primarily on continued progress in improving vaccine technologies and insights from large COVID-19 clinical trials.
Extraordinary advances in vaccine technology also came to fruition at just the right time. The Pfizer and Moderna vaccines both use mRNA technology that has been developed over the past 10–15 years. Scientists had developed ‘plug-and-play’ vaccines that act as ready-to-use platforms. When COVID-19 emerged, scientists plugged in the genetic code for a part of the coronavirus that would trigger our immune response.
Have you read?
The other vaccine that was one of the first to show effectiveness against COVID-19 was the Oxford-AstraZeneca vaccine that uses a viral vector – i.e. a different virus as the delivery system – to take genetic material that codes the SARS-CoV-2’s spike protein into our bodies. Viral vectors are not as new as mRNA vaccines, and it has taken years of research to identify the best vector that is both safe and ensures successful delivery of the vaccine antigen. In the case of the Oxford/AstraZeneca vaccine, a chimpanzee adenovirus that causes the common cold was chosen, and this was based on research in SARS, MERS-CoV and Ebola.
In addition to vaccine product development, manufacturing capacity was ramped up significantly in many countries. Although there remain supply bottlenecks and shortfalls, vaccine manufacturing capability is still much higher worldwide than it was pre-COVID-19.
Getting from lab to jab in 100 days
Radically reducing the speed of vaccine development will rely primarily on continued progress in improving vaccine technologies as well as insights from large COVID-19 clinical trials. These clinical trials are assessing different vaccine technologies in vast numbers of people, with different demographics and across various geographic locations. This data hasn’t ever been collected in such depth and it gives researchers a valuable resource to better understand immunity and how we respond to vaccines.
Safety and efficacy weren’t compromised for COVID-19 vaccine development, as several vaccine trials had independent data monitoring committees to review the data in real-time, rather than the usual lengthy post-development review process. For the next pandemic, innovations in review and regulatory approval are likely to allow the whole process to be further sped up.
The pandemic has also triggered changes in healthcare delivery that could be long-lasting. Telehealth or e-medicine has been around for several years, but hadn't been used to its full potential. COVID-19 has made telephone or video consultations standard and, though this was started as a way of avoiding unnecessary travel or contact between patients and doctors, it has great value for people who find it difficult to access healthcare for other reasons.
Similarly, the World Health Organization (WHO) has recommended that it would be worthwhile for countries to invest in community health workers. These are workers who are connected to the people and are able to take health services to people when they can’t travel. They can also flag signals of clusters of disease to health systems. Community health workers can be critical in pandemic management, including vaccine deployment, as they can also help ease hesitation in taking vaccines.
Thinking ahead to the next pandemic even as we are battling this one is necessary given that another pandemic is an evolutionary certainty. Nevertheless, many pandemics are preventable before they become pandemics: investing in preparedness from better surveillance and detection, faster responses, and improved sharing of information between countries is critical no matter how quickly we may be able to respond to a threat.