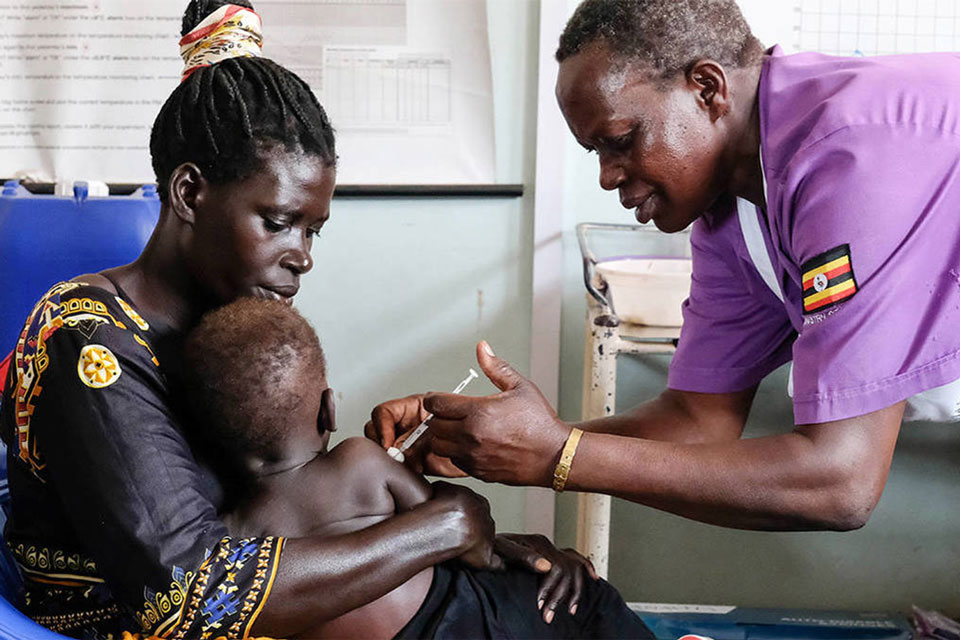
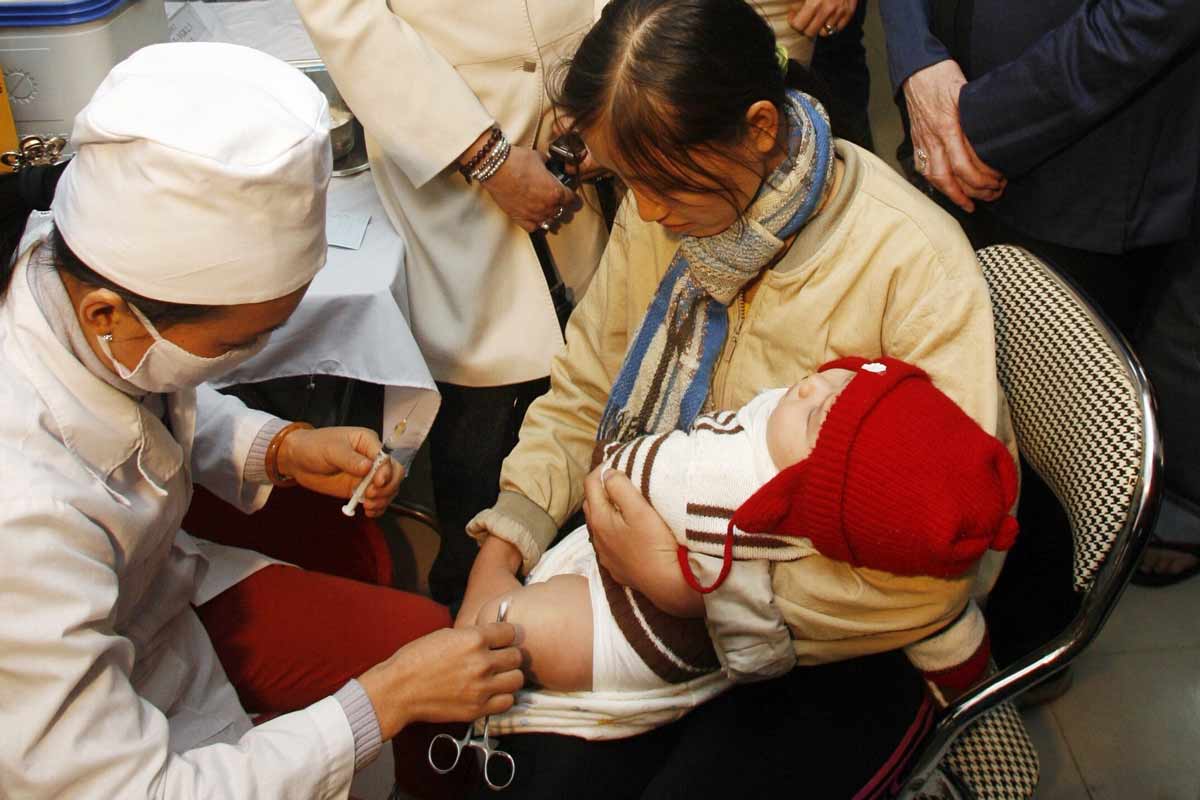
The Vaccine Alliance in 2023
Prof José Manuel Barroso, Chair of the Gavi Board, and Dr Sania Nishtar, CEO of Gavi since 18 March 2024, reflect on the driving forces of progress in 2023 and plans for 2024.
- 2 October 2024
- 4 min read
- by Sania Nishtar , José Manuel Barroso

Welcome to Gavi’s 2023 Annual Progress Report, the third of Gavi’s 2021–2025 strategic period (Gavi 5.0/5.1). 2023 marked the end of the COVID-19 pandemic and the launch of innovative tools to help ensure a rapid, equitable response to vaccine-preventable disease outbreaks. The Alliance intensified efforts to support countries in restoring routine immunisation and catching up missed children.
In 2023, global challenges meant Gavi had to intensify efforts to achieve its core priorities. The Vaccine Alliance remained adaptable, responding rapidly to country needs, seizing opportunities for impact, and maintaining efficiency, resilience and sustainability.
At the June 2023 Global Vaccine Impact Conference, hosted by the Government of Spain, world leaders convened to evaluate the Alliance’s implementation of the first two years of its 5.0/5.1 strategic period. The conference marked the release of Gavi’s Mid-Term Review (MTR) report, Raising Generation ImmUnity, showing that Gavi is “on track” to meet the majority of the key commitments made to donors.
Soon after, in August, Dr Seth Berkley completed his 12-year tenure as CEO. We commend Seth’s extraordinary contributions to global health over nearly four decades, and thank him for his unrelenting commitment to Gavi’s mission and building a strong foundation for future success.
In November, Cameroon became the first non-pilot country to receive doses of the RTS,S malaria vaccine – making history just two months later by launching the world’s first malaria vaccine as part of its routine immunisation programme. WHO’s October 2023 recommendation of a second vaccine, R21, will enable broader access and help meet strong country-led demand for malaria vaccines as part of holistic national malaria prevention strategies.
The need to strengthen how the world responds to evolving health risks shaped Gavi Board’s decisions at its December meeting in Accra, Ghana. The Board approved a series of landmark proposals covering pandemic preparedness, routine immunisation and the Alliance’s future. One of the key learnings from the COVID-19 pandemic is the urgent need for more equitable global distribution of vaccine manufacturing capacity. Accordingly, the Gavi Board adopted the principle of the African Vaccine Manufacturing Accelerator (AVMA), which aims to sustainably support the development of Africa’s vaccine manufacturing base with up to US$ 1.2 billion in funding over ten years. Similarly, the First Response Fund, with US$ 500 million for early‑stage global health security response, will enable a more rapid and effective response to pandemic risks as part of the broader Day Zero Financing Facility for Pandemics (DZF).
The COVAX Facility played a central role in enhancing global vaccination rates and vaccine equity during the COVID-19 pandemic, averting millions of deaths in lower-income countries. Its operations concluded on 31 December, and we express gratitude to all involved for their commitment to vaccine equity and saving lives.
Have you read?
The results in this report position the Alliance strongly to achieve the majority of Gavi 5.0/5.1 goals, noting that challenges persist. To combat cervical cancer, six Gavi‑supported HPV vaccine introductions in 2023 are crucial towards reaching our target of reaching over 86 million girls by end 2025. Progress towards restoring routine immunisation after the disruption of COVID-19 is mixed; and reaching the unreached remains a challenge: Gavi-supported countries remained at 80% DTP3 coverage in 2023, and there were 18% more ‘zero-dose’ children than in 2019, requiring a 37% reduction to reach the Gavi 5.0/5.1 target by end 2025.
While 2024 progress will be covered in next year’s report, much has already happened as this report goes to print: Dr Sania Nishtar joined Gavi as CEO on 18 March, immediately embarking on a 180-day plan supporting the year’s priorities and beyond. June saw the launch of Gavi’s Investment Opportunity to support its next replenishment during The Global Forum for Vaccine Sovereignty and Innovation, co-hosted by Gavi, the African Union and the French Republic. This forum underscored the importance of collaboration, solidarity and partnership with AVMA’s launch. Earlier that month, the Gavi Board approved the strategy for Gavi’s 2026–2030 strategic period (Gavi 6.0), making us optimistic about the potential for the Vaccine Alliance to continue driving positive change.