Executive summary
Once every five years, Gavi, the Vaccine Alliance carries out a detailed horizon-scanning exercise to assess the impact, cost, value and feasibility of expanding its portfolio to include new and under-used vaccines of most relevance in the countries it supports. The Vaccine Investment Strategy (VIS) enables Gavi to maximise the number of lives it helps save and the number of people whose health it improves, while facilitating long‑term planning for partners, donors, manufacturers and Gavi‑supported countries.
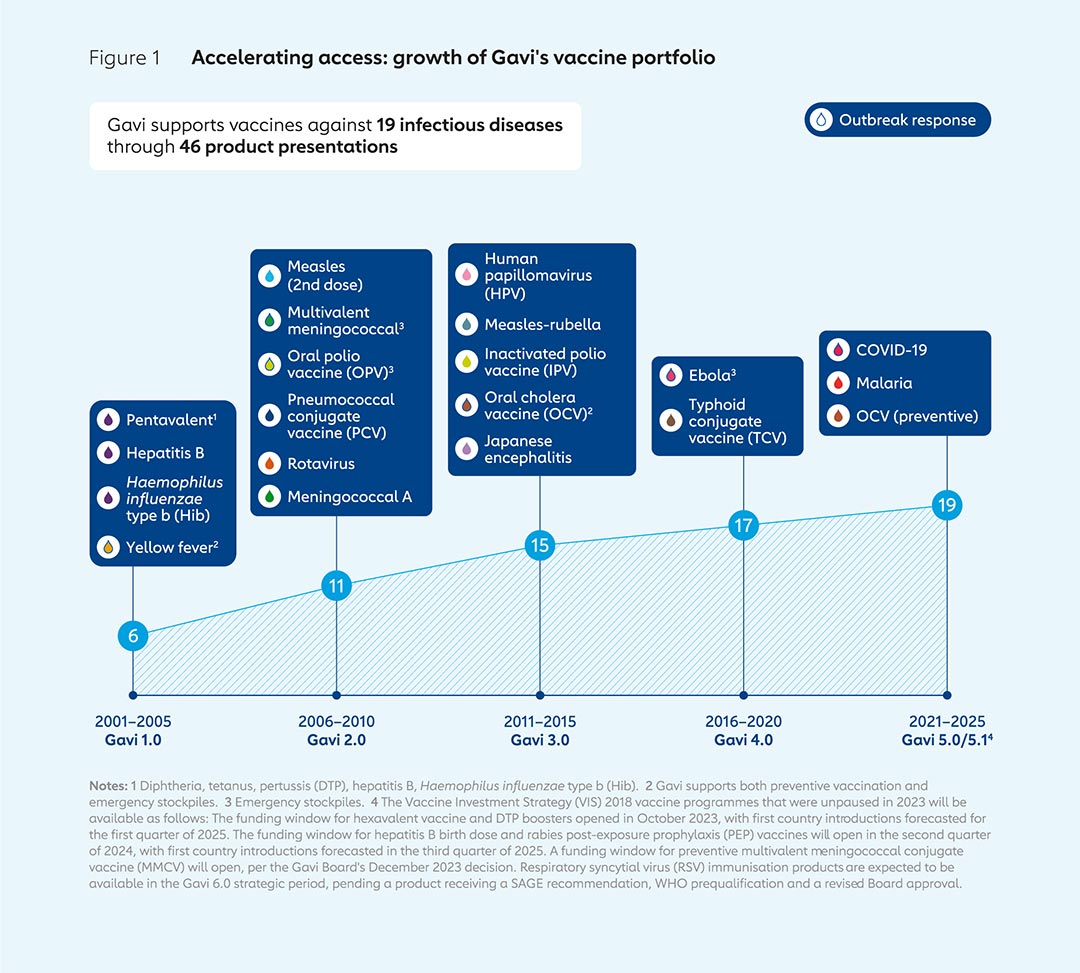
Today, Gavi supports vaccines to protect against 19 pathogens, up from 6 in the year 2001. During that period, more vaccines have been developed, and Gavi has widened its scope from its early focus on protecting infants to also supporting vaccines for those in other age groups, such as school-entry age and adolescents; as well as adults, for example through certain vaccines during outbreaks, epidemics and pandemics.
As the number of vaccines being developed has increased, so too has the complexity of the trade-offs needed to determine which new vaccines to support. These changes in the vaccine landscape have underlined the importance and value of the VIS in helping Gavi target valuable resources to achieve the greatest possible impact.
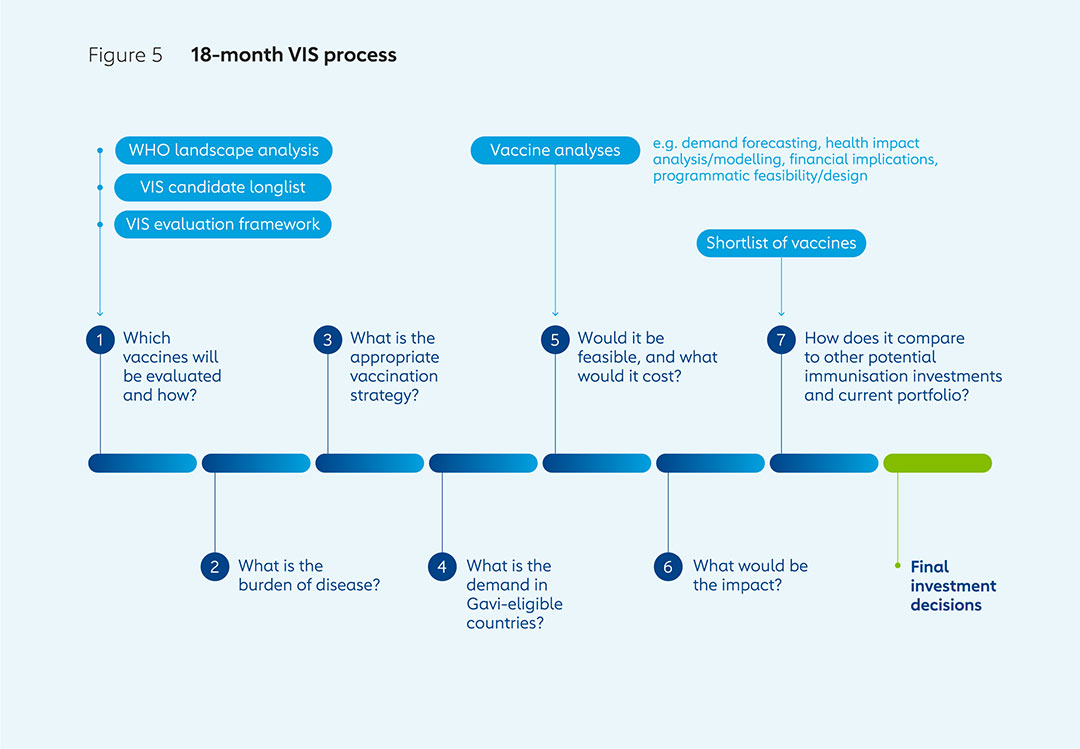
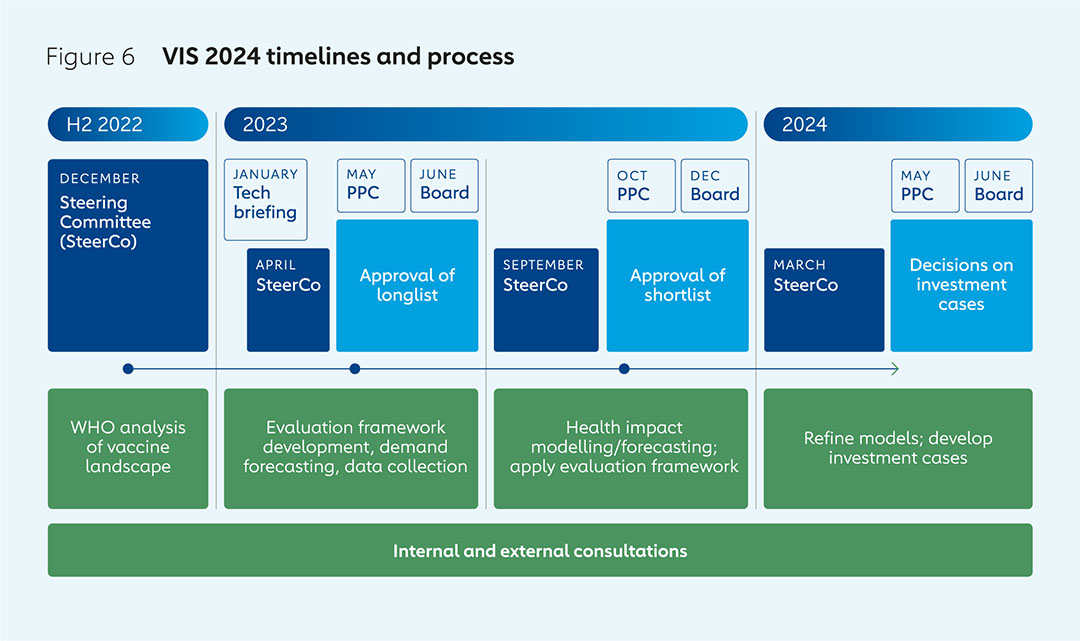
The VIS is an 18-month process that begins with the generation of a longlist of vaccine candidates with the help of the World Health Organization (WHO). Assessments are made of potential health impacts, economic implications, disease risks and Gavi’s ability to make an impact, leading to the drawing up of a shortlist. Detailed investment cases for shortlisted vaccines are then compared to enable final decisions. The VIS process is based on two different evaluation frameworks to assess potential investments for routine immunisation programmes and preventive vaccination campaigns, and those for outbreak response.
These frameworks are published, alongside the analysis the process is based on, to ensure the exercise is open and transparent. The VIS is highly consultative, taking into account the perspectives of implementing countries, donors, manufacturers, civil society organisations, academics, disease experts and others. It is also evidence-based, thanks to in-depth reviews of existing research, expert consultations and modelling work. The use and ranking of key criteria that are revised and clearly outlined at the start of the process make the VIS both rigorous and systematic.
The VIS changes over time to reflect emerging ways of assessing impact, new sources of data, Gavi’s strategic priorities and the global context. The methodology used in 2023–2024 has been revised in a number of ways, so that, for example, it takes into account climate change risks and mitigation, and improves the ways it assesses the impact of antimicrobial resistance (AMR). These changes, alongside the rigour, consultation, transparency and evidence-based principles on which the process is built, will ensure the VIS plays a key role in helping Gavi deliver on its mission for many years to come.
1. What is the Vaccine Investment Strategy?
Since its inception in the year 2000, Gavi, the Vaccine Alliance has helped vaccinate over 1 billion children and prevented more than 17.3 million future deaths by improving access to new and under-used vaccines in lower-income countries.
The Alliance works continuously to understand how the resources provided by donors and by implementing countries can be best used to save more lives, and protect the health and livelihoods of those in lower-income countries.
Every five years, Gavi takes stock of the vaccine landscape to enable it to better understand the potential impact, cost, value and feasibility of supporting the introduction of new and under-used vaccines. This process, which begins with an analysis of new immunisation products in collaboration with the World Health Organization (WHO), is called the Vaccine Investment Strategy (VIS). It enables Gavi to determine which vaccines and immunisation products to prioritise to maximise its impact and achieve its goals, including shortening the time between life‑saving vaccines being available in high‑income countries and being rolled out in lower‑income countries. The VIS also provides predictability and supports long‑term planning for partners, donors, manufacturers and Gavi‑supported countries.
When Gavi was launched, powerful new vaccines were becoming available, yet nearly 30 million children in lower-income countries were not being fully immunised against deadly diseases, and many were receiving no immunisation at all. Today, there are many more vaccines available, some of which have regional rather than global relevance, making the prioritisation process more complex at a time of financial constraints and global health security risks.
Based on VIS analyses, Gavi has increased its portfolio from vaccines against 6 infectious diseases during its 2001–2005 strategic period to 19 in 2022 through 46 different vaccine products. Over time, Gavi’s focus has shifted from initially supporting only routine infant vaccination to prevent endemic diseases, to also funding vaccines to protect those in other age groups, such as adolescent girls with HPV vaccine, for example; and to building stockpiles of vaccines to deploy during outbreaks, epidemics and pandemics, such as those used to combat yellow fever and Ebola. The development of new vaccines, including some with greater importance in some regions than others, has made Gavi’s task of determining which ones to prioritise for introduction into its portfolio more complex, underlining the importance of the VIS as a rigorous, evidence-based, consultative and transparent exercise.
The VIS is designed to encompass a full evaluation of the value of vaccines, including their impact on mortality and morbidity, as well as the social, economic and population health benefits. Shaped by the current global context and Gavi’s key strategic priorities, the process considers a wide range of factors, from costs and demand forecasting, to the most appropriate vaccination strategies and implementation feasibility in Gavi implementing countries. In this way, it enables comparisons and trade-offs between different new and under-used vaccines, based on key variables and benefits.  |  |
Preventing cervical cancer deaths in lower-income countries
Human papillomavirus (HPV) is the leading cause of cervical cancer, which caused more than 348,000 deaths worldwide in 2022. It is the fourth most common cancer in women, and more than nine in ten cervical cancer deaths occur in low- and middle-income countries.
Following extensive clinical trials, two HPV vaccines, which protect against the main types of HPV that cause cervical cancer, were licensed in 2006 and 2007. The vaccines were rapidly introduced in high-income countries, but roll-outs were limited in lower-income countries because of high prices and delivery challenges.
In 2008, the Gavi Board selected HPV, Japanese encephalitis, rubella and typhoid vaccines for support from a longlist of 18 diseases and pathogens provided by WHO, as part of the VIS. It also adopted the reduction of disease burden in Gavi-supported countries as a key strategic objective.
Detailed assessments were carried out of public health impact and value for money, based on demand forecasts, vaccine and system costs, feasibility, introduction challenges and risks relating to vaccine introductions. The analyses presented to the Gavi Board in 2011 indicated the HPV vaccine could be one of the highest-impact introductions Gavi could support based on the potential numbers of future cervical cancer cases and deaths averted, and the low cost of the vaccine.1
Gavi began supporting countries with HPV vaccines in 2012. It approved funding for demonstration programmes in 20 countries during 2012–2013 and for national introductions in three more: Rwanda, Uganda and Uzbekistan.2
Gavi launched efforts to accelerate the rate of national HPV vaccine introductions in 2016; however, global supply shortages slowed progress. COVID-19 also hampered HPV vaccination programmes, which reach the majority of girls in schools, many of which were closed for long periods during the pandemic.
Market shaping efforts have helped ease HPV vaccine supply constraints in recent years. By the end of 2022, Gavi had supported the full HPV immunisation of more than 16.3 million girls. In December 2022, the Gavi Board committed more than US$ 600 million to a revitalised and ambitious HPV vaccine programme aimed at reaching more than 86 million girls by 2025 and averting more than 1.4 million future cervical cancer deaths as a result. This initiative was supported by the easing of vaccine supply constraints and a new WHO position paper that indicated that one dose of vaccine confers significant protection. As of December 2023, 38 countries had introduced HPV vaccine into their routine immunisation programmes with Gavi support.3
2. How the VIS captures the full value of vaccines
The VIS process draws on the expertise of Alliance partners and a wide range of other stakeholders. It begins with an analysis of the vaccine landscape in collaboration with WHO. The Gavi Board makes the final decisions on investments based on advice and recommendations from the Gavi Programme and Policy Committee (PPC). A VIS Steering Committee, made up of independent experts with a wide range of expertise and observers representing Gavi partners, provides technical advice on the methodology and the various options for future investment.
The evaluation of candidate vaccines follows a process consisting of three phases, each of which culminates with decisions made by the Gavi Board:
Phase 1 involves generating a longlist of potential vaccine investments, starting from WHO’s analysis of immunisation products of relevance to Gavi-eligible countries that are not already in Gavi’s portfolio; are already licensed or are likely to be licensed; and are likely to be recommended for use by WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) by the end of the forthcoming five-year Gavi strategy.
Advice from the VIS Steering Committee and external consultations are used to shape the development of the two evaluation frameworks used to assess products on the longlist: one for investments in routine immunisation and preventive vaccination campaigns, and another for investments in outbreak response. Different frameworks are required, as there is more uncertainty involved in evaluating the potential benefits of stockpiling vaccines for use in response to outbreaks than for investments in those used as part of routine immunisation and preventive campaigns.
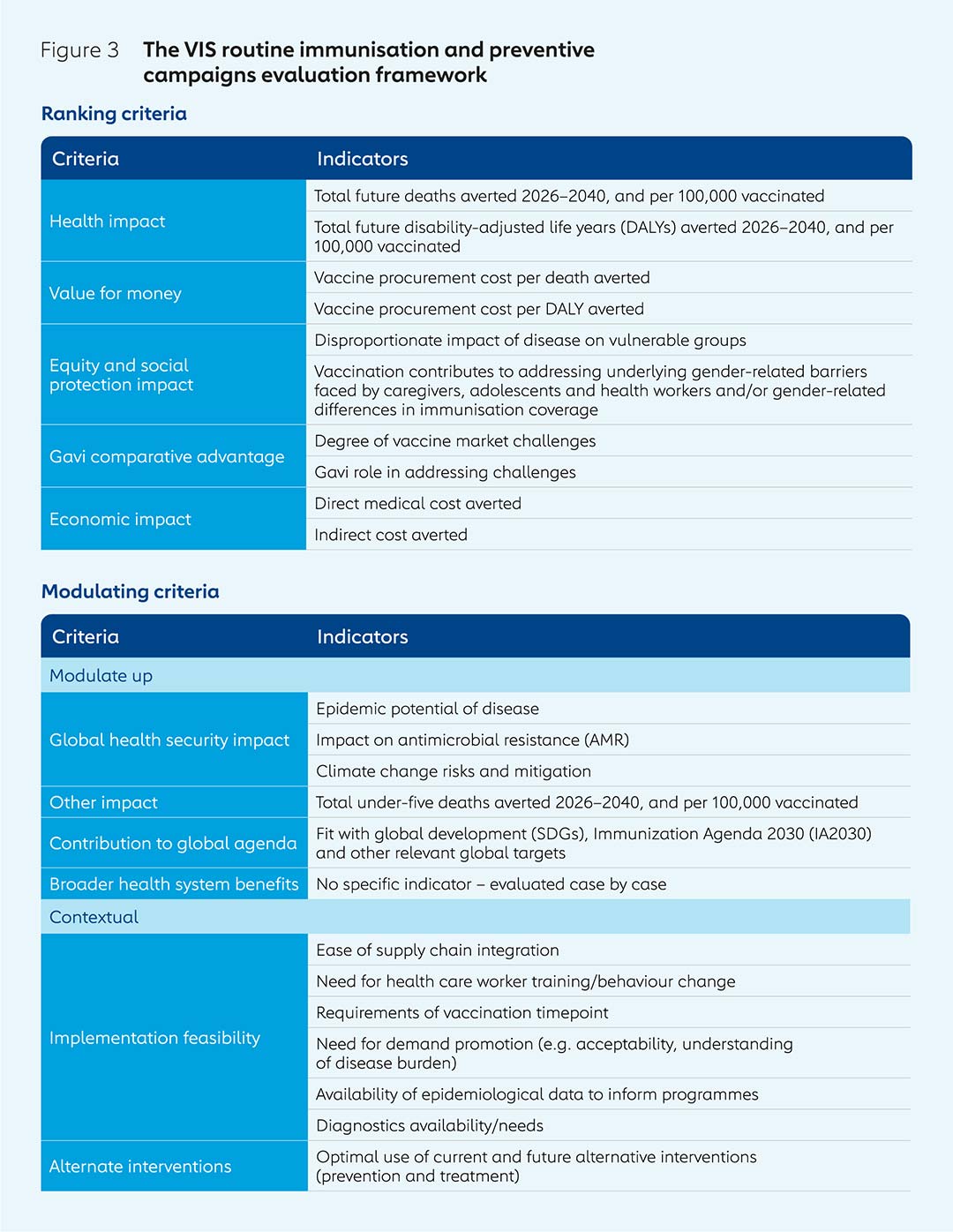
The routine immunisation evaluation framework is based on a series of ranking and modulating criteria, for which there are a number of key indicators in each case.
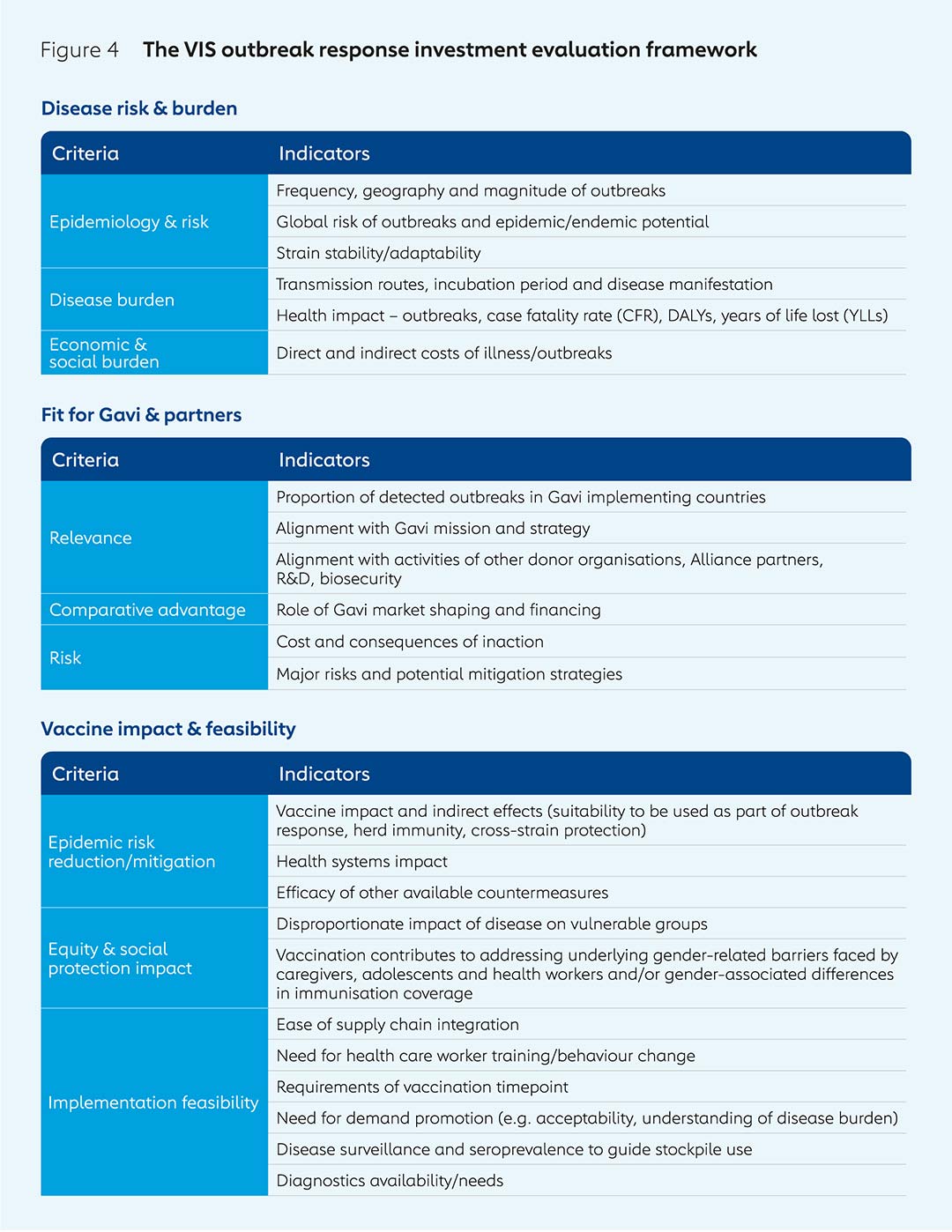
The outbreak response investment evaluation framework is, during phase 1, structured around three key areas: disease risk and burden; vaccine impact and feasibility; and how Gavi’s expertise can contribute to funding and delivery of a vaccine. Potential investments in vaccine products for protection in outbreaks, epidemics and pandemics can also be evaluated outside the standard five-year VIS cycle to enable agile decision-making in response to emergencies or research & development breakthroughs (see “Assessing the life-saving potential of malaria vaccines” below).
Phase 2 sees quantitative and qualitative analysis of each of the vaccines on the longlist, again based on evaluation frameworks for routine immunisation and outbreak response, and in consultation with academics, WHO and other experts. Disease profiles are investigated, including burden, pathogenesis, transmission, causes, symptoms, diagnosis and interventions. Information is gathered from experts and manufacturers on vaccine product characteristics such as supply and manufacturing capacity, price estimates, efficacy, safety, as well as their regulatory and policy status.
Evidence is also collected and generated through interviewing disease-specific experts on vaccination delivery strategies, including whether vaccines are expected to be delivered as part of preventive or outbreak response vaccination campaigns, or both. The most appropriate age (or other target population such as health workers or pregnant people) at which a vaccine product would be delivered, the number of doses and vaccine schedules are considered, aligned with a SAGE recommendation, if available, or otherwise with clinical trial data. The settings in which vaccines would be delivered are also factored in, such as health care clinics, maternity wards or mobile vehicles for campaigns in remote areas, for example.
Likely vaccine coverage is estimated as part of demand forecasting. This requires calculation of the proportion of target population groups who are likely to be reached in participating countries. Coverage predictions are easier when proxy vaccines can be used. For example, data on providing the second dose of measles-containing vaccine (MCV2) could be examined when considering coverage for a single-dose dengue vaccine, as recommended vaccination ages are close together. It can be more challenging for some vaccines for certain population or age groups when there are fewer or no established proxy vaccines, such as when considering providing a tuberculosis vaccine at the age of 15.
All this evidence, including vaccine demand and price forecasts, is fed into quantitative modelling run by academic modelling groups across a range of institutions that specialise in specific diseases to assess the potential impacts of vaccine investments. Outcome metrics include deaths, cases, years of life lost (YLLs) and future disability-adjusted life years (DALYs) averted by vaccination. These outcomes for the VIS vaccines are compared to those vaccines that Gavi already supports to understand if the potential impact is within the same range. This process forms a key part of the evaluation framework used to inform the narrowing down of the longlist to a shortlist.
Phase 3 involves the development of detailed investment cases for VIS shortlist candidates. The modelling of health impacts such as deaths and numbers of years lost to ill health or death, and economic impacts such as medical costs saved, is refined. So too is demand forecasting, through, for example, integration of better data on country introduction timelines. Consultations with disease experts, academics and others enable this process to provide more robust assessments of the potential impacts and cost of investments over the envisaged timeframes.
The feasibility of implementing new vaccination programmes is also considered during phase 3. This includes, for example, an assessment of how challenging it would be to reach age or population groups that are not currently part of existing vaccination programmes and services. It also takes into consideration the potential need to re-train health workers or ensure the necessary surveillance and diagnostics capacity to track disease epidemiology is in place.
Based on this in-depth additional evidence and analysis, the total cost of introducing vaccination programmes is calculated for each vaccine on the shortlist. This includes not just the costs of vaccine procurement, but also the costs to Gavi and implementing countries of any associated health system strengthening needed, such as new equipment, public awareness campaigns or integration into existing services such as antenatal care.
Alongside the investment cases for vaccine programmes, proposals for research on operational questions, such as how to ensure people return to receive all their doses or improving understanding of disease burden, are developed and prioritised. Market challenges and the potential for Gavi to address these through market shaping are examined. Lastly, an assessment of risks of both taking forward each vaccine programme and of not doing so is carried out. The combination of all this additional, in-depth information forms the comprehensive investment case for each vaccine that is presented to the Gavi Board to ensure it is well equipped to make the final key decisions on which vaccines to support at the culmination of the VIS process.
What next?
The decisions made by the Gavi Board may signal the end of the VIS process; however, there is still some way to go before new vaccines reach those they are intended to protect. There may or may not be a licensed vaccine available, and only once there is, and it has been recommended for use and prequalified by WHO, can Gavi fund a vaccination programme. This needs to be designed including, crucially, details of how the vaccine should be used, including information on target ages and dose schedules. Co-financing arrangements that outline the costs participating countries will be expected to pay and what additional support Gavi or others can provide must be determined. Once the details of a vaccination programme have been developed, a date from which countries can apply for funding can be set.
Q&A: Professor Helen Rees, Chair of the VIS Steering Committee
Professor Helen Rees was appointed Chair of Gavi’s VIS Steering Committee in October 2023. She is Executive Director of Wits RHI, at the University of the Witwatersrand in Johannesburg, South Africa, and chair of WHO’s African Routine Immunization Technical Advisory Group (RITAG). Wits RHI is a multi-disciplinary research institute that focuses on reproductive health, infectious diseases and vaccines.
How would you summarise the function and value of the VIS?
The VIS enables a diverse group of experts to consider which new vaccines Gavi should support over the next five-year period. The process reviews the burden of disease, the importance and feasibility of vaccine introduction in Gavi-eligible countries, and the potential public health impacts. Thinking this far ahead has been a progressive approach that facilitates market shaping and one that was ahead of its time when it was established more than two decades ago.
Why does the VIS process take 18 months?
The VIS involves consolidating and reviewing existing data, generating modelling evidence to assess impact and discussing the specific vaccines with other stakeholders. This provides an important level of objectivity. It’s an iterative and consultative process involving the Gavi Secretariat, the VIS Steering Committee, the Programme and Policy Committee (PPC) and the Gavi Board; and conversations with constituencies, such as countries for which vaccines might be relevant. The VIS needs to be highly rigorous because so much is at stake.
How does the composition of the VIS Steering Committee contribute to the process?
The Steering Committee members are researchers and experts who neither work for the Gavi Secretariat nor are current Gavi Board members. This allows the committee to review the data independently and provides a more objective, external perspective that helps build confidence in the process. The Steering Committee allows additional inputs from observers, other global organisations, donors and civil society organisations.
What do you personally hope to bring to the process following your appointment as chair of the VIS Steering Committee?
My view of the role of a chair is to listen, consolidate, feedback, steer and forge consensus among committee members. As Chair, my aim to is also to listen to the observers, and to the feedback from the PPC and the Board, to ensure we end up with outcomes that reflect a wide range of views.
What does success look like for the VIS Steering Committee?
If the evidence and recommendations that the Committee presents resonate with the PPC and the Gavi Board, which importantly includes ministers of health from Gavi-eligible countries, then we will have succeeded. The ultimate test of success, of course, is whether, during the five years to which the VIS relates, the recommended vaccines are successfully introduced and the anticipated health impacts are achieved.
3. Foundational principles at the heart of the VIS
The VIS is built on four core value pillars:
Consultation
From the generation of the vaccine investment longlist and shaping of evaluation frameworks, to the narrowing down to a shortlist and comparison of detailed investment cases, a wide range of stakeholders are consulted. Gavi-eligible countries are consulted through a variety of methods including surveys and interviews with government representatives, Expanded Programme on Immunization (EPI) managers and in-country Alliance partners. This provides information on the priorities of participating countries, likely vaccine demand levels and concerns around implementation feasibility, for example.
Civil society organisations are consulted on priorities and opportunities, and provide vital insights and data to inform the VIS process. Donors can provide information on levels of interest in funding new vaccines. Manufacturers also have important roles, providing key insights on pipeline vaccines, the potential for scale-up of products, vaccination strategies and intelligence on pricing.
Rigor
Potential vaccine investments are assessed based on frameworks established in advance. The current evaluation framework for vaccines for use as part of routine immunisation and preventive campaigns, for example, includes key criteria used to rank potential vaccine investments such as health impact and value for money; and modulating criteria that may or may not be relevant to each vaccine but can provide extra data, such as contribution to the global agenda and implementation feasibility. The methodologies used are clearly outlined and followed strictly to ensure evaluations are systematic and comprehensive.
Evidence
The VIS Steering Committee includes individuals with a wide range of technical and scientific expertise. Reviews of research literature are carried out to identify existing evidence. Consultations with academics, disease experts from partner organisations and the public health community provide further information. Data is generated through modelling exercises to assess the potential impacts of vaccine products and vaccination strategies. VIS data requirements are closely aligned with those of the WHO Full Value of Vaccine Assessments (FVVA) and Evidence Considerations for Vaccine Policy Development (ECVP). The use of the best-available evidence ensures investments are independent, objective and maximise returns in lives saved as well as social, health and economic benefits.
Transparency
The evaluation frameworks are freely available on the Gavi website, as are papers that are presented to the Board at various points during the VIS process. Gavi representatives attend academic conferences to share details of the methodologies and principles used.
4. Assessing the life-saving potential of malaria vaccines
An estimated 619,000 people died of malaria in 2021, of which two thirds were children aged under five in Africa.
Malaria vaccine was first considered as part of the VIS in 2013, at which time the leading vaccine candidate RTS,S/AS01e, developed by GlaxoSmithKline (GSK), was in phase III clinical trials. Modelling carried out by the Swiss Tropical and Public Health Institute and Imperial College London as part of the VIS suggested it had the potential to prevent more than 1 million future deaths in Gavi-eligible countries in Africa by 2030.
To fund the introduction of new vaccines, Gavi requires they are recommended for use and prequalified by WHO. This had not yet happened for RTS,S/AS01e in 2013, so the Gavi Board deferred a decision until a later date. In 2015, following the licensure of RTS,S by the European Medicines Agency (EMA), WHO advised that a pilot programme was needed before they could make a recommendation for broader public use. The following year, Gavi, the Global Fund to Fight AIDS, Tuberculosis and Malaria and Unitaid announced joint funding of US$ 72.4 million of the WHO-coordinated Malaria Vaccine Implementation Programme (MVIP) pilots, which were launched in 2019.
This caused uncertainty for GSK over whether it should continue production of the vaccine after all the doses needed for the MVIP were completed. So, in 2021, an innovative financing agreement was reached under which Gavi would fund GSK’s continued production of the RTS,S antigen, with MedAccess agreeing to pay for most of the costs if Gavi was unable to later approve a vaccination programme. Shortly after this, WHO announced its recommendation that RTS,S/AS01e be used in children in sub-Saharan Africa and other regions with moderate to high Plasmodium falciparum malaria, alongside insecticide-treated bednets and anti-malaria drugs.
The evidence and analysis on the potential impacts and costs of RTS,S/AS01e were further updated and presented to the Gavi Board in 2021, including a revised estimate that the vaccine could avert between 359,000 and 501,000 deaths by 2035. Growing evidence that antimicrobial resistance (AMR) and climate change were emerging as challenges to malaria control were noted as part of the VIS evaluation. The disproportionate impact of the disease on women and girls was also seen as an important consideration.
The MVIP pilots found the vaccine to be both safe and effective, reducing all-cause mortality by 13% in children in the age group eligible for vaccination and hospital admissions with severe malaria by 22% in the same age group. In December 2021, Gavi announced plans for an initial investment in malaria vaccine introduction, procurement and delivery. In late 2023, the cost of its malaria vaccine programme was estimated to be US$ 224 million for 2021–2025. Gavi forecasts suggest demand will reach 80–100 million doses per year by 2030, with at least 21 million children receiving the first of three doses in their first year of life and at least 15 million receiving a fourth dose in their second year of life.
More than 6 million doses of RTS,S/AS01e have been given to children in Ghana, Kenya and Malawi through the MVIP, and the first doses for routine vaccination programmes were shipped in November 2023.
While the VIS operates on a five-year cycle to provide predictability and enable long-term planning, Gavi also has the capacity to react rapidly to events, such as clinical trial results and WHO recommendations, to support new vaccine introduction programmes outside this cycle.
5. Updating the VIS for 2023–2024
Today’s vaccine landscape is more complex than it was 20 years ago, with more vaccines available, including those for age and population groups beyond those given as part of routine infant vaccination programmes.
Some new vaccines have been developed to combat diseases with moderate disease burdens or with only regional relevance, potentially posing difficulties for those seeking to bring them to market. Some countries may find it difficult to introduce new vaccines at a time when they are finding the implementation of existing vaccination programmes challenging.
The VIS must change with the times, and so it has been adapted to take into account both this increased complexity and the wider global context. The evaluation framework used for routine immunisation and preventive vaccination campaigns for the 2023–2024 VIS process has been updated in a number of important ways.
While the impact of proposed new vaccines on antimicrobial resistance (AMR) was previously based on qualitative interviews and consultations with experts, this time around new quantitative data and modelling are being used to enable more rigorous and accurate assessments.
The impact on climate change risks and mitigation has been introduced as a new indicator. It includes how climate change influences the potential geographical spread, incidence, frequency and severity of diseases for which new vaccine investments are proposed. It is being evaluated qualitatively through expert reviews, due to the lack of high-quality quantitative evidence on the likely changes in disease epidemiology due to climate change.
It is important to recognise the evidence gaps that can make it challenging to determine which new vaccine products are worth investing in. A lack of reliable disease burden data in some Gavi-supported countries can make estimating both vaccine impact and demand difficult. This is often the result of a lack of robust surveillance and, in some cases, of diagnostic capabilities. While the feasibility of implementation was already taken into consideration as part of the VIS, a new modulating criterion has been added for the 2023–2024 VIS to provide an assessment of existing disease surveillance and diagnostic capacity, as well as the potential for Gavi to address these challenges (see Figure 3).
Other changes include: new consideration of alignment with global development agendas and targets, such as the Sustainable Development Goals (SDGs); the addition of economic modelling outputs for endemic diseases, including treatment and delivery costs, indirect costs and cost-effectiveness; and reframing of equity and social protection impacts to capture a broader range of vulnerable groups and gender-related barriers to immunisation.
Also new for 2023–2024, the Gavi Secretariat carried out an assessment of the ethical values incorporated within the evaluation framework for routine immunisation, how they guide decision-making and whether there are any important gaps. Key substantive principles identified included: maximising health benefits and reducing harms; promoting equity by targeting disadvantaged groups; treating people with equal moral concern; and reciprocity, or preferential allocation to those who have taken on additional burdens to address health problems. Key procedural principles identified included: allocation based on transparency and evidence; genuine public engagement; and the regular review and revision of allocation guidelines based on evolving evidence of impact.
The ethical evaluation found that the VIS process in general and the evaluation framework for routine immunisation and preventive campaigns in particular address a significant number of these substantive and procedural ethical principles, and that others that are not explicit within the evaluation criteria, such as transparency, engagement and evidence responsiveness, are part of the wider VIS process.
Conclusion: harnessing the VIS to tackle current and future complexity
In global health, getting from key strategic objectives and ambitious targets to saving and improving large numbers of lives can be challenging. Maximising the impacts of interventions and overcoming the obstacles could hardly be more important when the goals are averting deaths, preventing disease cases and protecting livelihoods in lower-income countries.
The VIS plays a fundamental role by providing the roadmap that enables Gavi to identify the new and under-used vaccines with the greatest potential to save and improve lives in Gavi-supported countries and those that require significant financial support to do so. Its rigorous, evidence-based, consultative and transparent nature makes it the ideal process to help Gavi cross the bridge from aims to achievements.
The publication of VIS evaluation frameworks and data used in vaccine assessments provide rigour and transparency. The reviews of previous research, modelling exercises and input from scientists ensure the process is evidence-based. Use of evaluation frameworks based on scoring key ranking criteria and modulating criteria to generate a deep understanding of the full value of vaccines provides further rigour. Interactions with partner organisations, donors, countries, independent experts, civil society organisations, manufacturers and external observers makes the VIS highly consultative.
The importance of the VIS has grown over time. Compared to its early years two decades ago, Gavi has widened its mission beyond protecting only infants, and many more vaccines have been developed, some of which are mainly of regional importance. Making the necessary trade-off to determine which vaccines to invest in for maximum impact has, as a result, become more complex.
Looking forward, the number of vaccines can only increase, and the wider global health security context looks likely to add further complexity, which ultimately means the importance of the VIS is only likely to grow further still.
References
- https://www.gavi.org/news/document-library/06-next-steps-new-windows-pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4494350/
- https://www.gavi.org/our-alliance/strategy/vaccine-investment-strategy-2024








