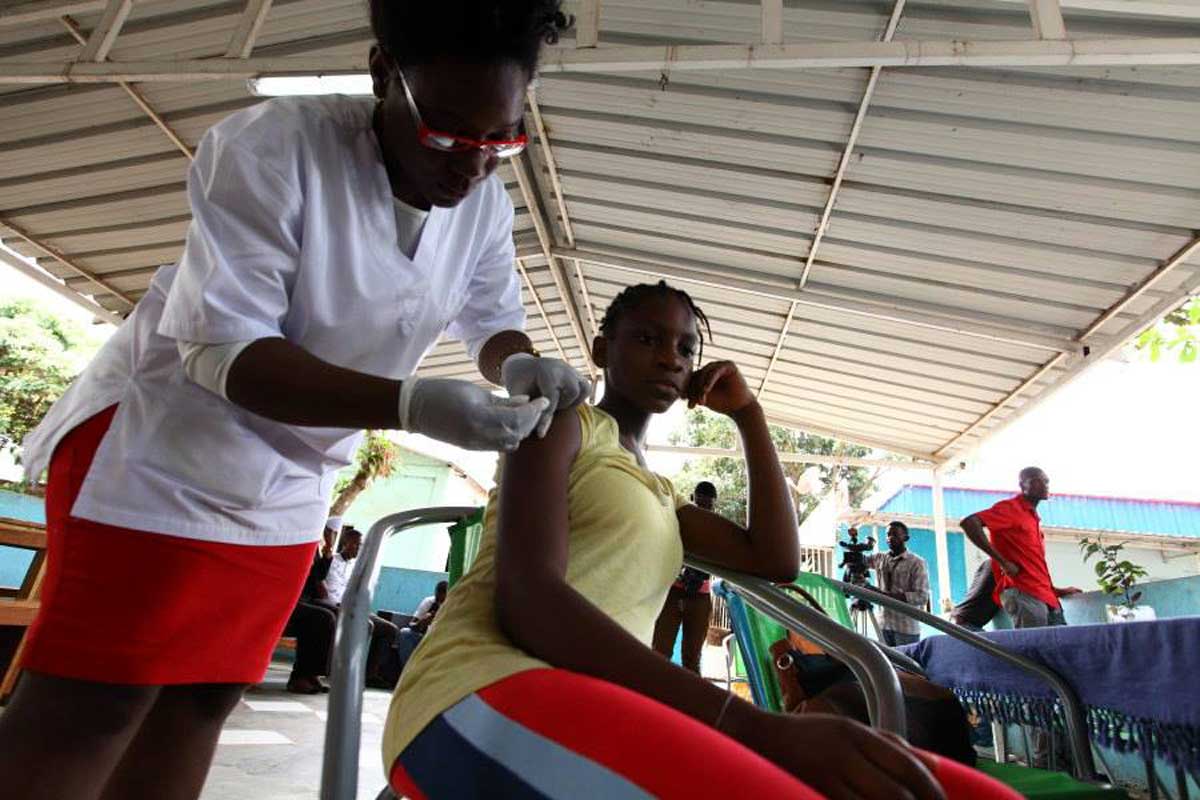
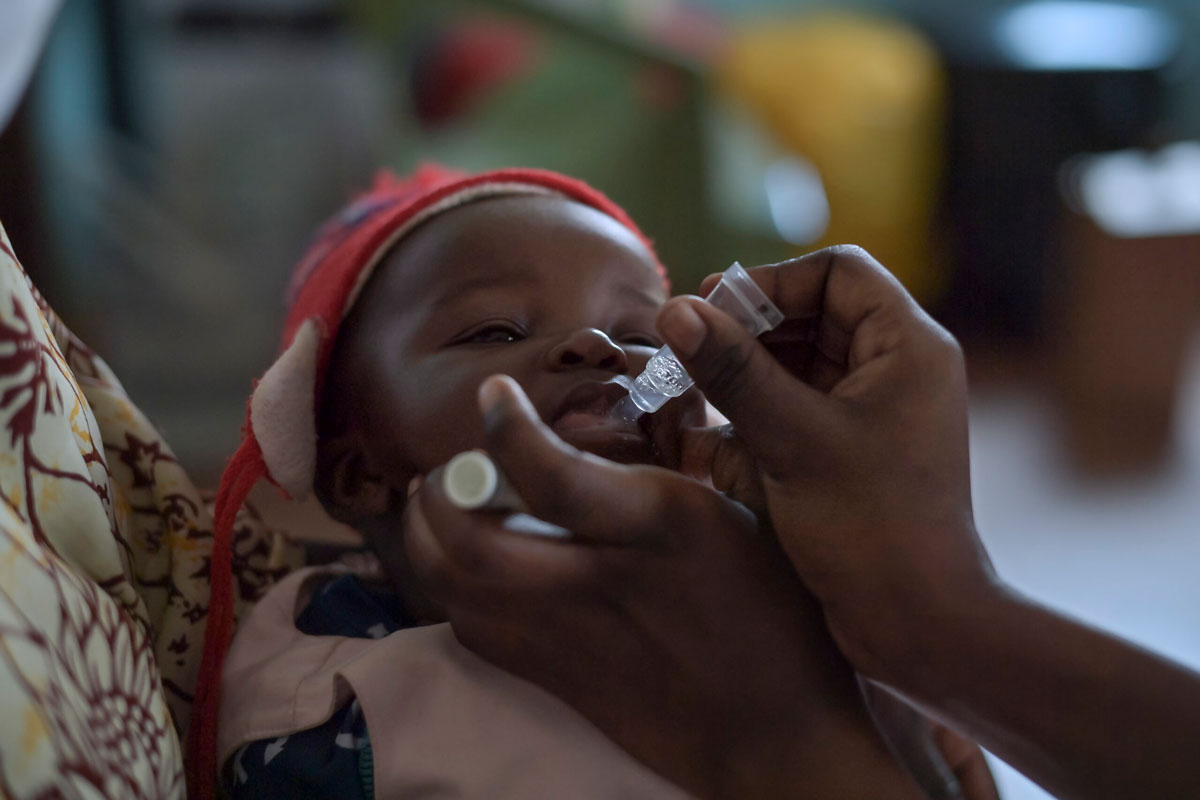
How will Cote d'Ivoire roll out the malaria vaccine?
Yesterday, Côte d'Ivoire made history by becoming the first country to introduce the R21 malaria vaccine into its routine immunisation programme. We spoke to the country's EPI Director to find out how they plan to roll out this lifesaver.
- 16 July 2024
- 6 min read
- by Assa Samaké-Roman

At the heart of this intense preparation, Dr Yao Kossia, Deputy Director of the Expanded Programme on Immunization, stands before the refrigerators filled with the crucial vaccines, ready for distribution to the initial districts.
We spoke to Dr Kossia ahead of the launch to gain an inside look at the roll-out of this groundbreaking initiative, exploring the implementation details of the new vaccine, the strategies in place to inform the public, and the logistical and financial challenges the programme faces.
Can you describe the scale of the malaria problem in Côte d'Ivoire, especially in terms of incidence and mortality, and its impact on families?
Malaria represents a significant public health challenge in Côte d'Ivoire. From 2018 to 2021, the incidence rate in the general population rose from 155 per 1,000 to about 300 per 1,000. For children under five, the situation is even more dire, with an incidence rate of approximately 600 per 1,000 in 2021.
In terms of mortality, over 1,000 children under five die from malaria each year in Côte d'Ivoire, which equates to around three child deaths per day. This highlights the severity of the problem and its impact on public health.
Until now, our prevention strategies have included the use of insecticide-treated mosquito nets, chemoprevention with sulfadoxine-pyrimethamine for pregnant women, and environmental sanitation efforts to control malaria vectors. However, the effectiveness of these measures is increasingly compromised by the growing resistance of malaria vectors to insecticides, which has escalated from 25% to 80%.
The consequences of malaria extend beyond health, affecting education and economic productivity. School and work absenteeism due to malaria-related illness results in significant loss of earnings, with households estimated to lose about 43% of their monthly income because of the disease.
The introduction of the malaria vaccine will be a crucial complementary measure to enhance existing prevention efforts. It aims to significantly reduce the incidence and prevalence of malaria, thereby improving overall health outcomes and alleviating some of the broader socio-economic impacts of the disease.
Can you outline the initial steps for introducing the new malaria vaccine?
For the initial phase of the malaria vaccine roll-out, we have identified 38 high-incidence districts. This will be the first stage of our routine introduction. Once launched, this vaccine will be incorporated into the existing routine immunisation programme. Thus, in addition to the current vaccines administered daily in health centres, the malaria vaccine will be offered to children in these selected districts.
The target group for this vaccine is children aged 0 to 23 months, with administration beginning at 6 months of age. Starting Monday, July 15, 2024, children from 6 months up to 23 months will begin receiving this vaccine, which is given in four doses. The schedule is as follows: the first dose is administered at 6 months, the second dose at 8 months, the third dose at 9 months, and the fourth and final dose at 15 months.

For children who start the vaccination schedule later than 6 months, the timing of subsequent doses will be adjusted accordingly. We strongly encourage parents to follow the recommended vaccination schedule to ensure their child receives all four doses, as these are crucial fo effective protection.
I would also like to emphasise that this vaccine is both safe and effective. It has been thoroughly tested and is provided free of charge.
Have you read?
What strategies are you implementing to inform the public about the new malaria vaccine?
We have developed a multifaceted communication approach to raise awareness about the new malaria vaccine. At the central level, we are using mass communication methods, while locally, each health district is engaging in targeted outreach. Recently, we conducted a briefing for local media presenters, bloggers, online press and community leaders to ensure widespread dissemination of information.
Our communication efforts will include interpersonal engagement at health centres, where more personalised information will be provided to parents as the vaccination campaign progresses.
Malaria is a serious and well-known disease, and it is crucial for parents to trust the health services. This vaccine has been successfully used in several other African countries, demonstrating its safety and effectiveness. Although the vaccine is provided free of charge to those who receive it, it is important to recognise that its development and distribution involve significant costs. The state and its partners have made substantial efforts to make this vaccine available, and I encourage all parents to take advantage of this opportunity to protect their children.
The vaccine has been shown to effectively reduce severe cases of malaria and lower mortality rates among young children.
What are the main challenges you face for the roll-out of the malaria vaccine, particularly in terms of district coverage and financing?
One of the primary challenges we face is programmatic. We are starting the vaccination roll-out in 38 districts out of 113, which means that 75 districts will not have immediate access to the vaccine. Given the scale of the programme and the communication efforts involved, there's a risk that neighbouring districts might feel left behind, which could complicate our efforts. To address this, we are focusing on raising awareness and ensuring that these districts understand that they will be included in future phases of the roll-out.
Funding is another significant challenge. While we receive crucial support from Gavi, we are approaching the end of our eligibility for their financial assistance. We are actively working on plans and strategies to secure state funding to cover the costs once Gavi’s support concludes.
We are optimistic because we have put several mechanisms in place to ensure that funding and vaccine distribution can continue seamlessly.

In terms of coordination and monitoring, we have established a comprehensive framework. An Interinstitutional Coordination Committee oversees the expanded vaccination programme, including the new malaria vaccine. This committee will closely monitor the performance of the vaccine roll-out and, if issues arise, it will provide recommendations to enhance the programme's effectiveness. This structured approach ensures that we can address challenges promptly and maintain the overall success of the vaccination initiative.
How is funding for the new malaria vaccine ensured, and what is the role of the different partners in supporting this vaccine?
The introduction of this new malaria vaccine is supported through a collaborative funding model. Gavi plays a key role by covering a significant portion of the vaccine's cost. The State of Côte d'Ivoire also contributes financially, sharing the cost burden. In addition to the vaccine purchase, there are various operational expenses involved, including training for health care workers, communication efforts, and supervision to ensure that the vaccine is administered according to established guidelines. Gavi provides crucial financial support for these aspects as well.
Beyond Gavi, other partners such as the World Health Organization (WHO), UNICEF and the President's Malaria Initiative (PMI) are integral to the programme. These organisations offer technical support and help mobilise additional resources. While Gavi and the State cover part of the budget, these partners work together to fill any gaps, ensuring that we have the necessary funds and expertise to implement the vaccine effectively. Their combined efforts help address the full spectrum of needs, from procurement and distribution to on-the-ground implementation and monitoring.