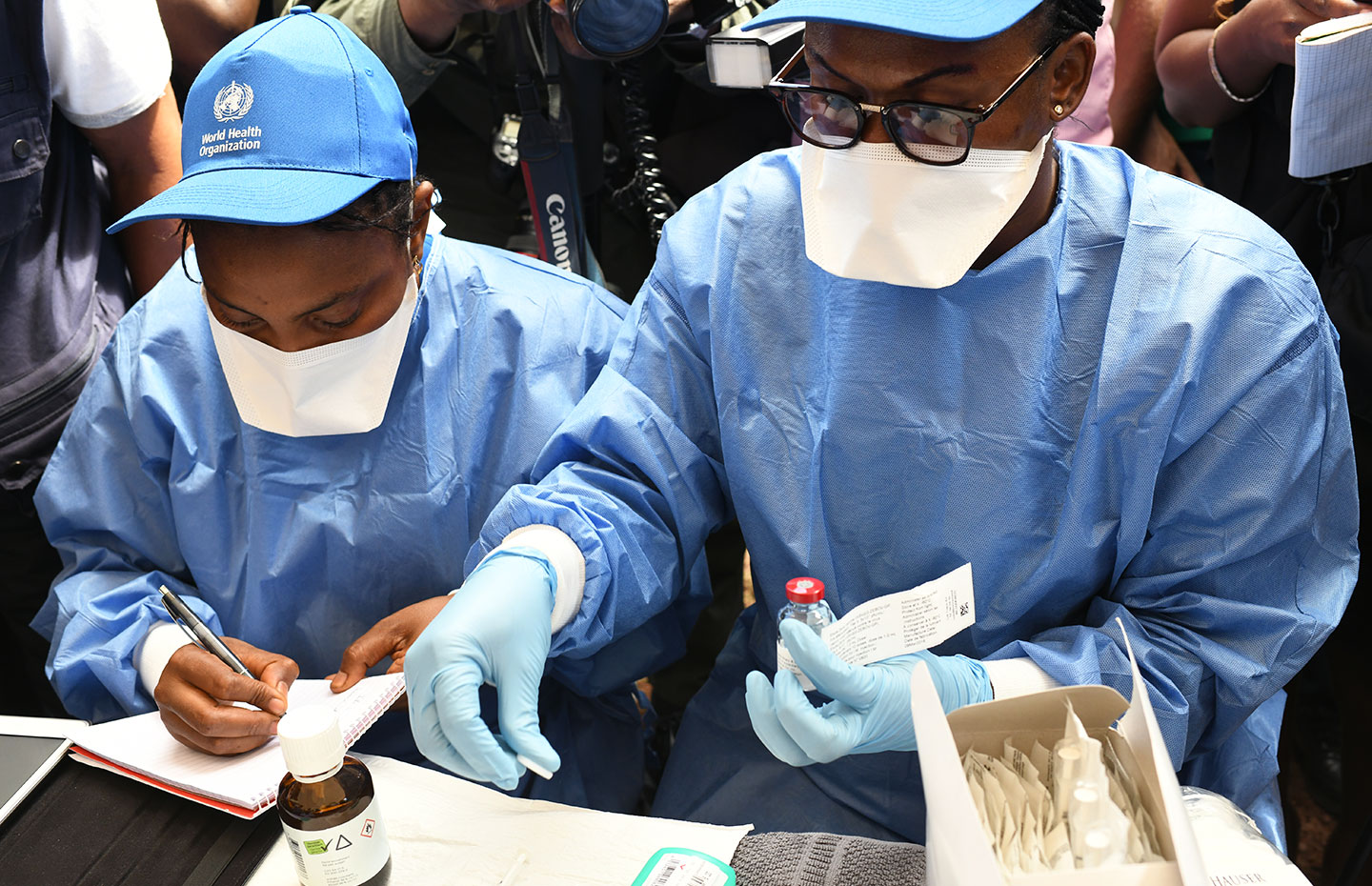
Geneva, 12 November 2019 – Gavi welcomes the European Commission’s conditional marketing approval for Ervebo (rVSVΔG-ZEBOV-GP), the first vaccine with clinical efficacy to protect individuals 18 years of age or older at risk of infection with the Ebola virus.
“This is a vaccine with huge potential,” said Dr Seth Berkley, CEO of Gavi the Vaccine Alliance. “It has already been used to protect more than 250,000 people in the DRC and could well make major Ebola outbreaks a thing of the past. That’s why this is such an important milestone, paving the way for a Gavi-supported global Ebola vaccine stockpile. It’s also important to credit the unprecedented global effort from African countries that helped generate the evidence as well as Merck, WHO, donor governments, partners and regulatory agencies in making this authorisation happen.”
At its next meeting in December 2019, the Gavi Board is set to make a decision on a long-term Gavi Ebola vaccine programme that would include the creation of a global Ebola vaccine stockpile. This stockpile, contingent on the availability of World Health Organization (WHO) prequalified vaccines and SAGE recommendations for their use, will enable countries to access and rapidly deploy Ebola vaccines in response to outbreaks. On top of the stockpile, the Board will also consider, if recommended, future support for preventive vaccination of high-risk groups outside of an outbreak such as healthcare workers in countries classified as being at high risk.
Data from clinical trials and compassionate use protocols have shown that Ervebo protects against Ebola virus disease in humans following a single dose administration. In the ongoing Ebola outbreak in the Democratic Republic of the Congo (DRC), the vaccine is being used under ‘compassionate use’ to protect people, including children and pregnant women, at highest risk of infection as part of a ring vaccination strategy, as well as a targeted geographical vaccination when ring vaccination is not feasible. As of 11 November, more than 250.000 people have been vaccinated in DRC, as well as in Burundi, Uganda, South Sudan and Rwanda.
The current stockpile of Ebola investigational vaccine is available in part thanks to an agreement between Gavi and the vaccine’s manufacturer, Merck. In 2015, during the West Africa Ebola outbreak, Gavi made a unique offer to all manufacturers with a vaccine in Phase I clinical trials and beyond, by offering a pre-paid commitment to procure doses of licensed vaccines as and when the vaccine became licensed and available.
In return, the manufacturer was required to commit to submit an emergency use authorisation application to WHO as well as an application for licensure to a stringent health authority and ensure the availability of an emergency stockpile of 300,000 investigational doses in the event of an outbreak occurring before licensure through a donation from the manufacturer to WHO. In January 2016, an Advance Purchase Commitment between Merck and Gavi was signed, creating the stockpile of investigational doses that is being used in DRC and neighbouring countries today.
In addition to its work making the investigational vaccine stockpile available, Gavi has provided US$ 15.1 million to WHO to cover operational costs for the vaccination effort, funding vaccination teams, transportation, syringes and other vaccine supplies, as well as the ultra-cold fridges which keep the vaccine at the minus 60-80°C temperatures it needs to remain effective. This also includes US$ 2 million provided to fund vaccinations in neighbouring countries Uganda, South Sudan, Burundi and Rwanda. An additional US$ 13.4 million funding for the vaccination effort in DRC is currently being considered.
Notes to editors
About Gavi, the Vaccine Alliance
Gavi, the Vaccine Alliance is a public-private partnership that helps vaccinate half the world’s children against some of the world’s deadliest diseases. Since its inception in 2000, Gavi has helped to immunise a whole generation – over 760 million children – and prevented more than 13 million deaths, helping to halve child mortality in 73 developing countries. Gavi also plays a key role in improving global health security by supporting health systems as well as funding global stockpiles for Ebola, cholera, meningitis and yellow fever vaccines. After two decades of progress, Gavi is now focused on protecting the next generation and reaching the unvaccinated children still being left behind, employing innovative finance and the latest technology – from drones to biometrics – to save millions more lives, prevent outbreaks before they can spread and help countries on the road to self-sufficiency. Learn more at www.gavi.org and connect with us on Facebook and Twitter.
The Vaccine Alliance brings together developing country and donor governments, the World Health Organization, UNICEF, the World Bank, the vaccine industry, technical agencies, civil society, the Bill & Melinda Gates Foundation and other private sector partners. View the full list of donor governments and other leading organizations that fund Gavi’s work here.