-
Gavi’s investment in initiative to boost yellow fever diagnostic capacity across Africa has ‘revolutionised’ diagnostics on the continent reducing the risk of future epidemics
-
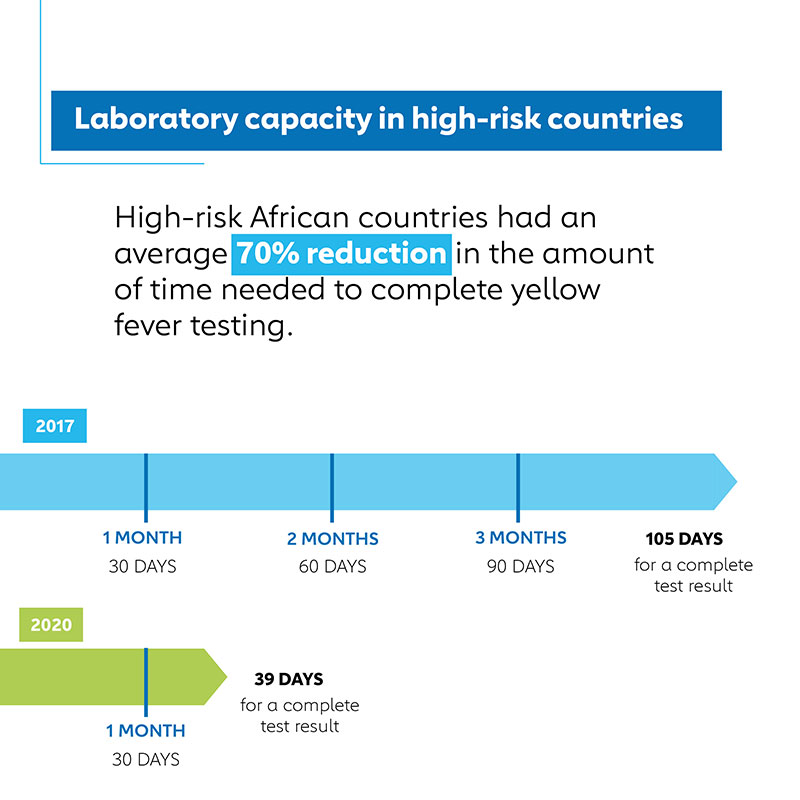
From 2017 to 2020, African countries at high risk of yellow fever epidemics have seen a 70% reduction in the amount of time needed to complete testing
-
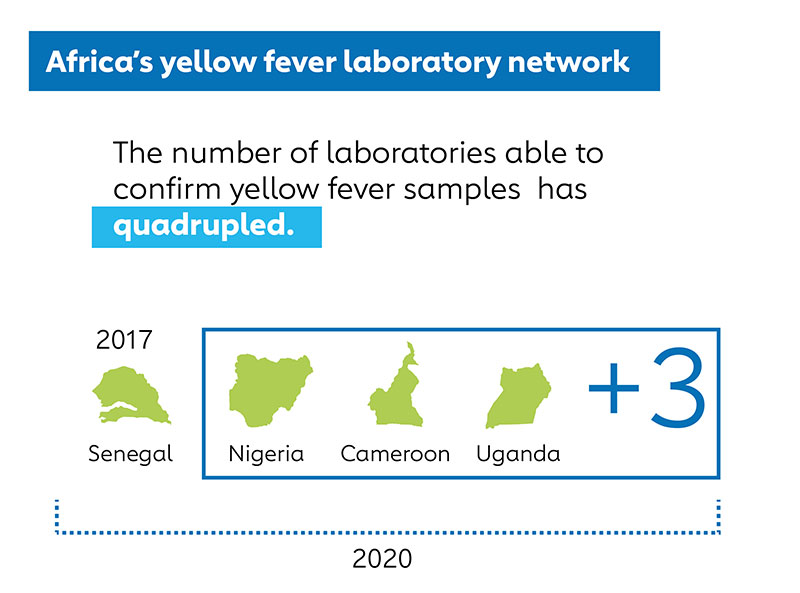
The number of laboratories able to confirm yellow fever samples on the continent has quadrupled and the first commercial yellow fever PCR testing kit is now available
Geneva, 7 September 2021 – An initiative to boost yellow fever diagnostic capacity across Africa has ‘revolutionised’ diagnostics on the continent, one of the programme’s coordinating agencies, Gavi, the Vaccine Alliance, said today.
The initiative is part of the implementation of the Eliminate Yellow Fever Epidemics (EYE) strategy and brings together different partners including WHO, UNICEF, CDC, the Institut Pasteur Dakar, Centre Pasteur Cameroon, and the Uganda Virus Research Institute. The diagnostic capacity initiative has made significant progress since its launch in 2018:
- The number of laboratories able to confirm yellow fever samples on the continent has risen from one, in Senegal, to four, with Nigeria, Cameroon and Uganda now able to definitively determine if someone has yellow fever, particularly early in the course of illness. This laboratory network has helped save nearly US$ 2 million in unnecessary spending by correctly identifying disease cases and allowing public health authorities to make timely decisions that minimise waste. In addition, data from this network has driven country decisions to protect their populations against yellow fever in the long term, such as Uganda’s decision in 2020 to introduce the yellow fever vaccine into routine immunisation.
- Chronic shortages of laboratory supplies that impeded timely yellow fever testing have been resolved by the establishment of testing bundles that can be readily ordered by national laboratories. The average time for national laboratories to complete the testing of samples initially positive for yellow fever confirming whether a new outbreak has started or an existing one has expanded, has declined by 70%, from over three and a half months in 2017 to 39 days in 2020.
- A new commercial PCR test kit validated by the EYE laboratory technical working group is now available for use in national laboratories that are part of the WHO yellow fever laboratory network.
“Prior to this investment in diagnostic capacity, it was a huge challenge for countries across Africa to accurately ascertain where yellow fever outbreaks were at risk of breaking out,” said Dr Seth Berkley, CEO of Gavi, the Vaccine Alliance. “This lack of capacity not only meant outbreaks, such as the 2015 epidemic that hit Angola and DRC, were able to spread rapidly before containment measures could be put in place, it also meant expensive precautionary, yet unnecessary, vaccination campaigns may have taken place in areas where cases were in fact low. In just a few years this investment has revolutionised capacity in a number of countries, putting them in a far better position to tackle this terrible disease.”
This progress is in part due to a new pooled procurement mechanism to buy yellow fever test kits and obtain critical testing supplies, helping to shape a commercial market for these crucial diagnostic tools. UNICEF Supply Division created new supply routes and relationships with manufacturers to improve the availability and distribution of testing supplies, while the EYE laboratory technical working group developed specifications and evaluated commercial test kits to identify which ones were accurate and reliable enough to be worth using.
“The expansion of UNICEF’s valued partnership with Gavi, to include diagnostics has already borne positive results and I am happy to see the boost in yellow fever diagnostic capacity in Africa,” said Etleva Kadilli, Director of UNICEF Supply Division. “Building on market and procurement expertise UNICEF and partners will be contributing to closing the gap in yellow fever diagnostics in Africa and supporting countries to maintain their diagnostics programmes at a healthy pace.”
Dr Mike Ryan, Executive Director, WHO Health Emergencies Programme said: "The success of the EYE Strategy to date also lies in the continued investment of time and resources by our partners, such as Gavi, the Vaccine Alliance. For example, we see tangible improvements in our international samples' transportation time, meaning less time is taken between detection of yellow fever and response, which ultimately saves more lives. The investment in technical assistance and training to laboratories across the African continent, and in innovative diagnostic approaches moves us closer to our goal of eliminating these deadly epidemics, which burden Africa and the Americas, and have potential for international spread."
The initiative has also improved the transportation of samples, with the WHO working with shipping companies to set up an international sample transportation system and training logisticians across the continent. WHO, CDC, regional reference laboratories and others have provided technical assistance and training to increase the number of laboratories able to confirm yellow fever, including training in newer PCR and CDC-developed serologic test kits for yellow fever, and supporting capacity-strengthening work by national public health institutes.
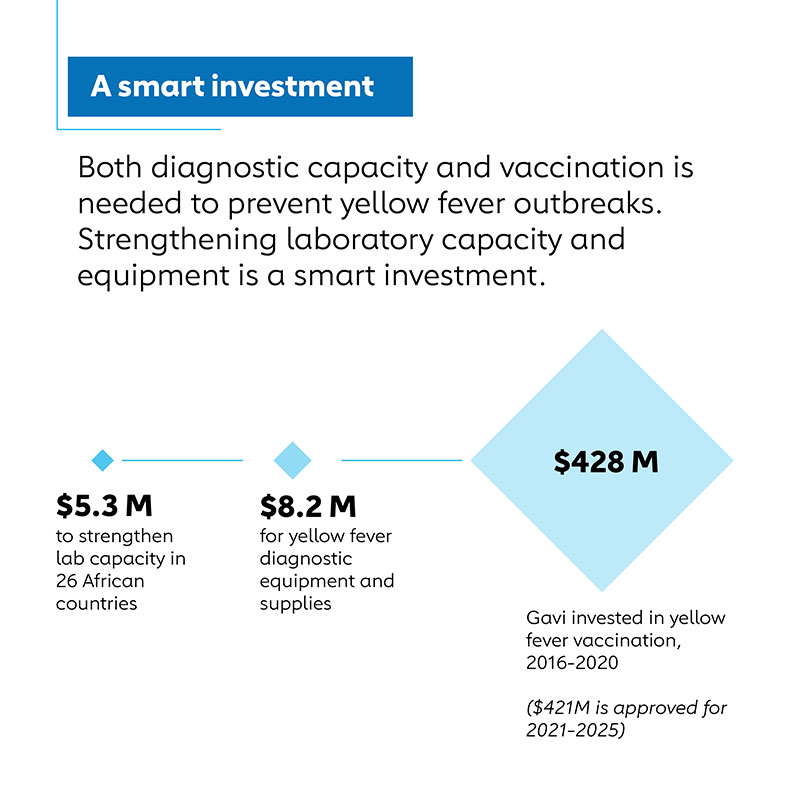
In 2018, the Gavi Board approved US$ 8.2 million in funding for yellow fever diagnostic equipment and supplies, as well as US$ 5.3 million to strengthen lab capacity in 24 African countries for the 2019-21 period. This funding for diagnostic testing was in support of much larger Gavi investments in yellow fever vaccination, such as US$ 428 million approved for 2016-2020 and US$ 424 million approved for 2021-2025, as part of the global Eliminating Yellow Fever Epidemics (EYE) strategy.
“Even though some of the most disruptive outbreaks of yellow fever occurred in the 1700s, it still presents a persistent and ongoing risk to public health, as was seen with the recent outbreaks in Angola and Brazil,” said Dr Erin Staples, a medical epidemiologist with the Arboviral Diseases Branch of U.S. Centers for Disease Control and Prevention. “Gavi’s investments in yellow fever vaccines are protecting millions of people. Their investment now in laboratory capacity has improved our ability to provide timely detection of disease cases and ultimately will help reduce the risk of yellow fever epidemics in the future.”
Yellow fever, a mosquito-borne viral haemorrhagic fever that can cause uncontrolled bleeding and death, clinically resembles many other diseases, such as Ebola virus disease and hepatitis A, B, and C. As a result, accurate diagnostic testing is essential for determining whether someone has yellow fever or another disease. Waning immunity and a decline in mosquito control efforts are shifting the geography of yellow fever, and the virus could now affect areas previously considered non-endemic. Recent yellow fever epidemics, which in some cases have involved travellers who carry yellow fever back to their home countries, underline the continued global threat posed by the disease.
Given the death and disruption that yellow fever can cause, diagnostic testing to improve the effectiveness, efficiency, and equity of yellow fever prevention through immunisation is the most cost-effective solution available.
Media Contacts
Meghana Sharafudeen
+41 79 711 5554
msharafudeen@gavi.org
Iryna Mazur
+41 79 429 3671
imazur@gavi.org
Cirũ Kariũki
ckariuki@gavi.org
Evan O’Connell
+33 6 17 57 21 26
eoconnell@gavi.org
Laura Shevlin
+ 41 79 529 92 87
lshevlin@gavi.org

