Summary
By the time the World Health Organization (WHO) characterised COVID-19 as a pandemic on 11 March 2020, teams of scientists across the world were already racing to develop vaccines to mitigate its impacts. COVAX, co‑led by Gavi, the Coalition for Epidemic Preparedness Innovations (CEPI), WHO and UNICEF, was established to handle the vaccines pillar of the unprecedented global response to COVID-19.
Gavi’s early focus was on the pooled procurement and equitable distribution of COVID-19 vaccines to ensure lower-income countries were not left out in the race to secure doses. The COVAX Facility was established by Gavi in April 2020 as a risk-sharing mechanism to achieve this goal. Other partners, donors and multilateral organisations focused on channelling financial resources to support country‑level delivery and logistics. This is atypical of Gavi’s usual model, which is to bundle delivery support with procurement support. Given the unprecedented scale of the crisis, Gavi originally planned to provide support for delivery only in exceptional circumstances.
Getting vaccines from manufacturing sites into people’s arms is a complex process even in normal conditions. It requires cold or frozen transport and storage; data monitoring; managerial capacity; health care worker training; public information campaigns and much more. These challenges are magnified and more difficult to overcome during a pandemic. While lower-income countries already had routine immunisation systems in place at the start of the pandemic, these predominantly focused on children. All countries were faced with a need to deliver many more vaccine doses than normal, and many would have to do so despite not having existing adult vaccination programmes in place.
Concerns grew during 2020 that even once COVID-19 vaccines had been developed and procured, gaps in the systems and processes needed to deliver them would prevent their successful roll-out in lower-income countries. In October 2020, the World Bank approved financing of US$ 12 billion for lower-income countries to buy and distribute COVID-19 vaccines, tests and treatments. However, it soon became clear that many countries were struggling to access sufficient and timely funding. Against this backdrop, COVAX partners developed a broad toolkit of support for the roll-out and scale-up of COVID-19 vaccination programmes in lower-income countries.
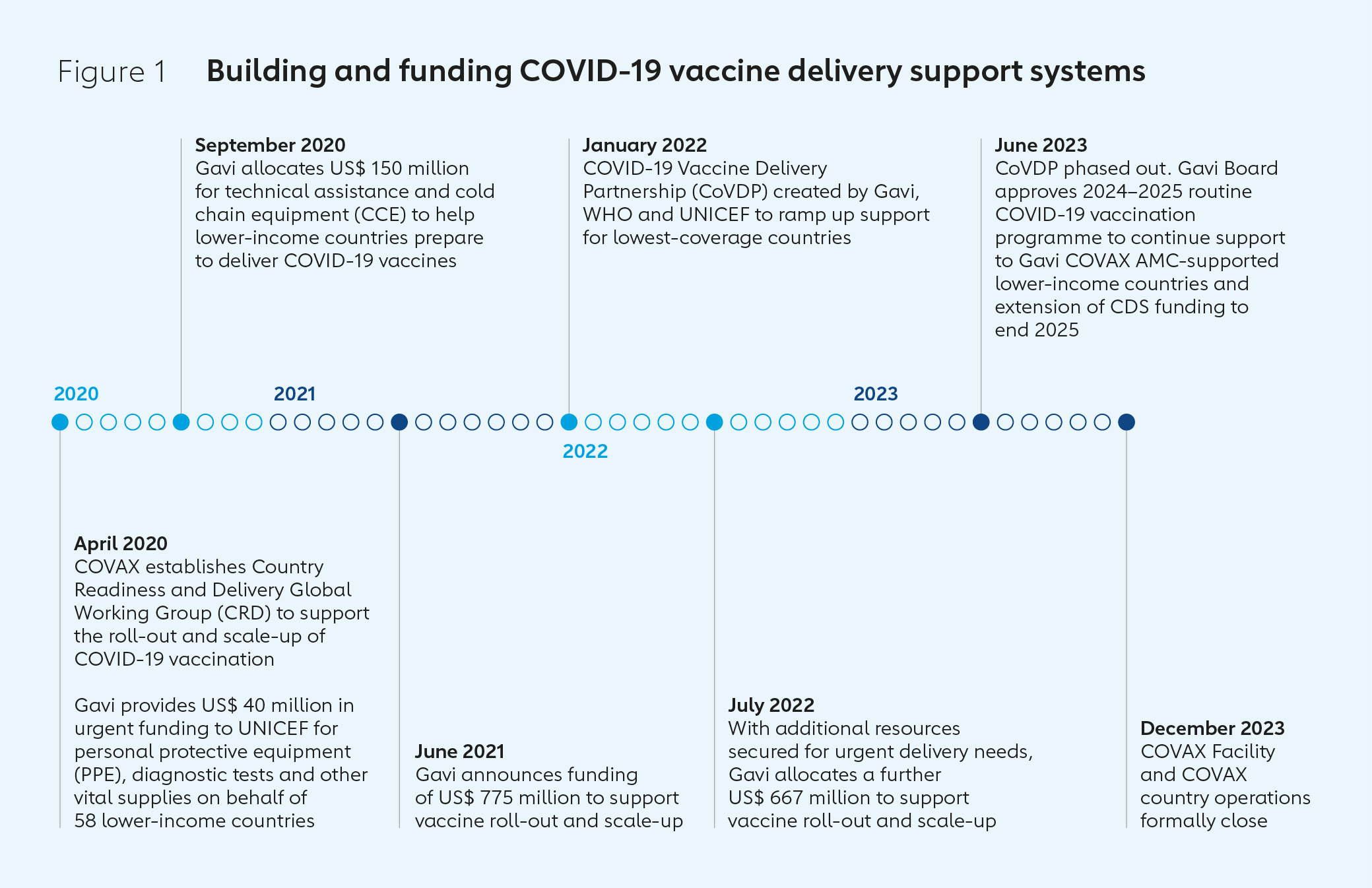
This analysis paper describes the circumstances in which the Gavi Secretariat took on a broader set of roles and responsibilities in supporting COVID-19 vaccine delivery than originally intended and summarises the nature of those contributions. It seeks to complement the efforts of the COVID-19 Vaccine Delivery Partnership (CoVDP) – which was created by WHO, UNICEF and Gavi in January 2022 – and others in assessing what worked well, what could have been done better and what should be done differently to make sure that vaccines required to deal with future international health emergencies are delivered both effectively and equitably to all who need them.
The COVID-19 pandemic has been devastating, with WHO reporting more than 750 million confirmed cases of COVID-19 and almost 7 million deaths.1 Others put the death toll much higher. The Economist estimates that, up to 15 June 2023, there had been 23.8 million excess deaths due to COVID-19 globally.2 It could, however, have been far worse. It has been estimated that COVID-19 vaccines averted 13.3 million deaths by the end of 2022 in the 92 low- and middle-income countries eligible to have their participation in the COVAX Facility funded by the Gavi COVAX Advance Market Commitment (AMC) financing instrument. More than one in five of these deaths – more than 2.7 million – are estimated to have been averted by COVAX-delivered vaccines.
COVID-19 put immense pressure on the health care systems of countries of all income levels and, in many cases, these systems demonstrated remarkable resilience. This is in part thanks to the work by Gavi with its partners and other organisations in lower‑income countries for more than 20 years to strengthen not just immunisation programmes, but also the underlying health care infrastructure needed to deliver them – from health and stock management information systems and logistics, to cold chain equipment (CCE) and health care worker training. Without these foundations, COVID-19 vaccine delivery would have been much more difficult in many low- and middle‑income AMC countries, and the pandemic would have caused significantly more loss of life and health, as well as economic damage.
The investments made by Gavi and COVAX to support COVID-19 vaccine delivery have helped strengthen the foundation of immunisation and health systems delivery in participating countries. Additional capacity built to deliver doses, including hard-to-reach populations and adults, are helping countries catch up on routine immunisation and will help underpin further expansion of delivery, as well as resilience against pandemics and emergencies in the future. Infrastructure and equipment investments made during the pandemic are similarly supporting other aspects of health care, while vaccine technology innovations developed as part of the fight against COVID-19 are already being applied more widely.
There is also greater awareness of the political, economic and moral cases for ensuring everyone has access to immunisation and effective health care more broadly, thanks to advocacy and communications work during the pandemic. Gavi is working with its partners and other organisations to maximise the health impacts of the investments made during the COVID-19 pandemic. The dividends in lives saved, illness prevented, health systems strengthened and health improvements of many types will be seen in lower-income countries for many years to come.
1. Preparing to deliver COVID-19 vaccines
During the 20 years before COVID-19, the Vaccine Alliance supported governments in vaccinating more than 822 million children in 77 countries against diseases such as measles, diphtheria and polio. By the end of 2019, countries had prevented more than 14 million deaths with Gavi-supported vaccines. Gavi has long recognised the importance of health system strengthening (HSS) in achieving its objectives through improvements in areas including supply and cold chains, health management information systems, leadership and management, and technical assistance in lower-income countries. Gavi has significantly increased the funds it has distributed to support HSS over the years, from US$ 315 million in the 2006–2011 strategic period (Gavi 2.0) to US$ 1.4 billion in 2016–2020 (Gavi 4.0).
Discussions between leadership at Gavi and CEPI on the need for equitable distribution of any successful vaccines against COVID-19 began in January 2020, when the potential scale of the health emergency was beginning to be recognised. As a result, a discussion paper on potential scenarios and considerations for a fair vaccine allocation system was circulated between the two organisations in February. This document went on to help inform the creation of COVAX.
WHO characterised COVID-19 as a pandemic on 11 March 2020. By then, there had been more than 118,000 cases in 114 countries and close to 4,300 deaths. As the virus spread rapidly across international borders, it became clear that COVID-19 threatened to overwhelm health systems in countries of all income levels, putting at risk many of the gains of the previous two decades.
At this time, Gavi highlighted the risk that COVID-19 could cause significant falls in routine immunisation coverage and increase risks to frontline workers and health services more broadly. It also threatened major economic shocks that could undermine the ability of countries to pay for immunisation services. At its March 2020 meeting, the Gavi Board approved a plan for Gavi to work with CEPI, WHO, World Bank, UNICEF and other partners to accelerate equitable access to vaccines. The Board also agreed a proposal to introduce flexibilities to Gavi’s operating model, such as waiving co-financing requirements and allowing countries to redirect existing funding from its health system and immunisation strengthening (HSIS) programme for critical COVID-19 responses such as surveillance and providing personal protective equipment (PPE) for health care workers.
The work of Gavi, CEPI and WHO towards equitable access to COVID-19 vaccines was incorporated into a broader global health response with the launch of the Access to COVID-19 Tools Accelerator (ACT-A) in April 2020. COVAX, co-led by Gavi, CEPI, WHO and UNICEF, was established to handle the vaccines pillar of ACT-A, with other global health agencies focusing on access to diagnostics and therapeutics. With significant resources announced by the World Bank and other donors for vaccine delivery, Gavi and COVAX originally planned to provide financial support for delivery only in exceptional circumstances.
As case numbers and death tolls grew across the world, it became clear that coordination, training and guidance for vaccine delivery support would be essential to ensure the effectiveness of any eventual vaccination campaigns in lower-income countries. In April 2020, COVAX launched the Country Readiness and Delivery Global Working Group (CRD) workstream, led by Gavi, WHO and UNICEF, to support the roll-out and scale-up of COVID-19 vaccination programmes through: the development and dissemination of resources such as guidance, training, tools and communications materials; regular data monitoring; the provision of technical assistance in areas such as analysis of funding and supply chain logistics; and the promotion of coordination and collaboration between partners at global, regional and national levels.
Preparing countries for vaccine delivery required putting a variety of processes and mechanisms in place, including some that had never been used before. For example, COVID-19 vaccine manufacturers required those procuring and delivering vaccines to take on indemnification related to novel vaccines. Gavi recognised humanitarian agencies would be unable to do so and negotiated indemnity and liability waivers with manufacturers. COVAX introduced a no-fault compensation programme for the lower-income countries eligible for the Gavi COVAX Advance Market Commitment (AMC) – an innovative vaccine financing mechanism, largely funded by donor governments, designed to ensure rapid, fair and equitable access to COVID-19 vaccines. The no-fault compensation programme enables eligible individuals in AMC-eligible countries to receive compensation for adverse events associated with COVAX-distributed vaccines. Gavi also established an independently verified and assured equitable vaccine allocation framework.
In September 2020, the CRD launched the Vaccine Introduction Readiness Assessment Tool (VIRAT) to support countries in developing roadmaps to prepare for vaccine introduction and identify gaps that might require additional support. In the same month, Gavi approved US$ 150 million to fund additional technical assistance and cold chain equipment (CCE) in AMC countries.
In November, the VIRAT tool was combined with the World Bank’s Vaccine Readiness Assessment Framework (VRAF) to provide a way to assess whether countries were ready to deploy COVID-19 vaccines. Gavi pledged to work with the World Bank, CEPI and WHO to help at least 100 countries get ready to deploy COVID-19 tests, treatments and vaccines over a 100-day period. It also worked with partners to assist countries in preparing national deployment and vaccination plans (NDVP) for COVID-19 vaccines, which they could use when working with the the World Bank and the COVAX Facility.
By late 2020, multiple COVID-19 vaccine trial preliminary successes were emphasising the importance of vaccines as a means to get through the pandemic. The administration of the first dose of a COVID-19 vaccine approved by regulators following a large clinical trial took place at University Hospital, Coventry, UK, on 8 December. While India became the first country to administer a COVAX vaccine on 19 January 2021, Ghana became the first country to receive COVID-19 vaccine doses shipped internationally by COVAX on 24 February 2021.
In the following six weeks, COVAX shipped doses to 100 countries, but shipments slowed considerably following India’s decision, in response to a domestic COVID-19 surge, to halt exports of one of the few vaccines that had received WHO Emergency Use Listing. As those in higher-income countries began receiving their first doses, vaccine nationalism emerged as the biggest threat to equity, and it became clear that lower-income countries would fall behind in their efforts to protect their populations if they were unable to access the support they needed to undertake what would become the biggest vaccine roll-out in history.
2. Taking on a greater role in vaccine delivery
The lack of vaccine supply and continuing uncertainties around when larger volumes of doses would become available presented critical barriers to COVID-19 vaccine delivery and planning in early and mid-2021. As COVAX partners worked to resolve these issues by signing new advance purchase agreements and launching calls for dose donations, the lack of supply was compounded by a lack of resources to prepare for eventualities. CRD monitoring underlined growing concerns that even once greater quantities of vaccines did become available, their successful roll-out would be put at risk in lower-income countries by a lack of timely and sufficient funding. Many donors made clear their desire and commitment to provide support as the scale of potential delivery problems became apparent.
In a document published as part of the Gavi COVAX AMC Investment Opportunity of April 2021, Gavi outlined some of the ways it was turning its attention to working with partners on aspects of delivery. This included harnessing Alliance expertise in demand forecasting to provide vaccine manufacturers confidence in demand scenarios, as well as in related financial and supply needs. Gavi also outlined plans to help strengthen countries’ vaccine delivery systems ahead of a scale-up in roll-out by ensuring their CCE and technical assistance needs were met, as well as by scaling up high-impact innovations and providing greater delivery oversight to mitigate key risks. Against this backdrop, governments and private sector partners made pledges of more than US$ 2.4 billion and committed to donate millions of COVID-19 vaccine doses to COVAX for lower-income economies at two virtual events in April and June 2021. These were co-hosted by the governments of the United States and Japan respectively, and brought together world leaders, the private sector, civil society groups and technical partners.
The World Bank had approved financing of US$ 12 billion for lower-income countries to purchase and distribute COVID-19 vaccines, tests and treatments in October 2020. Yet by 4 June 2021, the World Bank had only approved 25 countries’ COVID-19 vaccine projects. It was clear a flexible approach with a higher risk threshold was required to address the challenge, and that few organisations were positioned to play this role.
In June 2021, following on from the Investment Opportunity event, the Gavi Board stepped in to fill this gap – approving a greater Gavi role in supporting COVID-19 vaccine programmes in AMC-supported countries to ensure populations in lower-income countries were protected. It announced the creation of a COVID-19 vaccine Delivery Support (CDS) funding package of US$ 775 million to support the roll-out and scale-up of COVID-19 vaccines.
During 2021, Gavi opened two CDS funding windows, alongside other financial support made available by WHO and UNICEF. The first, opened in July, provided US$ 252 million to catalyse countries’ early vaccine delivery efforts (the CDS1 Early Access funding window). The second, initiated in October with a budget of US$ 260 million, was for countries with the greatest needs and gaps in delivery funding (the CDS2 Needs‑based funding window).
The urgency of the public health need, combined with the uncertain environment, required increased risk tolerance. Funds were released under the first CDS window using existing risk mitigation mechanisms used in emergency and humanitarian contexts on a ‘no regrets’ basis. Subsequently, Gavi sought to ensure the timely release of funds to support delivery, while also conducting assessments of the risk of the potential misuse or duplication of financing. Gavi introduced risk mitigation approaches it hadn’t previously deployed, such as expanded use of third‑party monitoring agents. The intensive engagement of Alliance partners and Gavi senior country managers also helped navigate these challenges.
By the end of 2021, a total of 92 funding applications under the two CDS windows had been approved, and more than US$ 240 million either released or committed to AMC countries. Delivery problems began to ease in late 2021, as vaccine supply increased. The release of further CDS funds ramped up considerably in early 2022.
The largest deployment of ultra-cold chain equipment in history
Scaling up access to COVID-19 vaccines was not just a question of shipping containers of doses. It also required ensuring they were transported and stored at the correct temperature so that they were still effective when they were used. COVID-19 vaccines were distributed by plane, truck, motorcycle and even by foot to places with only basic health care infrastructure and some of the most remote places in the world. Previous Gavi investments in standard (2–8°C) cold chain equipment (CCE) prior to 2020 meant that the countries traditionally supported by Gavi had strengthened cold chain capacity, although it still needed to be expanded in many cases to meet the scale of the challenge. Non-AMC countries had not benefitted from this targeted investment, and most of the funds requested as part of the first CDS funding window were for CCE. Almost none of the 92 lower-income AMC-supported countries had the ultra-cold chain (UCC) equipment needed to store mRNA vaccines at the required -60°C to -80°C. Here, the Alliance’s experience with the Ebola vaccine, which has a similar cold chain profile, provided valuable learnings.
During the pandemic, 73 low- and middle-income countries received support to increase their cold and ultra-CCE and storage capacity. COVAX’s response amounted to the largest deployment of UCC equipment in history; a major undertaking that included the assessment of country requirements as well as the financing, coordination and delivery of 948 UCC freezers to nearly 70 countries during 2021 and 2022.
UCC equipment is significantly more complex to install and manage than standard CCE; and while Gavi provided funding and coordination, Vaccine Alliance founding partner UNICEF led the way on the delivery of UCC equipment. Initial deliveries were targeted at countries first receiving the Pfizer vaccine, because it required UCC storage.
The UCC deployment also required: building up vaccine management capacity, including through the deployment of specialists; outsourcing the installation and maintenance of equipment; infrastructure investments such as electric power stabilisation and back-up power supply; and overcoming logistical challenges, such as transporting large and sometimes dangerous equipment by air at a time of global transport disruption.
Adapting through dose redeployment
In late April 2021, an aeroplane left the Democratic Republic of the Congo carrying half a million doses of COVID-19 vaccines. These had been supplied to the country by COVAX in early March, but the government alerted COVAX it would be unable to administer them before their expiry date. Those half a million doses were part of a consignment of 1.3 million doses redeployed to other African nations: Angola, Central African Republic, Ghana, Madagascar and Togo.
These 1.3 million doses were a precious commodity in a time of scarcity, and the redeployment was possible because the Congolese health minister knew COVAX would prioritise ensuring these vaccines were used to protect people elsewhere, and the country could recover its allocation at a later date, when it was better prepared to administer them. It was a precursor to a formal COVAX vaccine redeployment policy, launched later in 2021 amid the broader supply-constrained environment, to support countries wanting to ensure doses they could not use did not go to waste. Almost 2 million doses were redeployed in this way.
This experience also helped inform the process of allocating and shipping doses, particularly dose donations, that came with short notice and often with short shelf lives: COVAX worked with the African Union to set clear minimum standards, and ensured dose donations to COVAX were only accepted once a country, with full knowledge of all the relevant information, agreed to take them on.
The redeployment policy was suspended in April 2022 because by then supplies had greatly increased and countries had greater visibility of demand, so the transfer of short shelf-life doses between participants was no longer needed, and the associated quality risk, administrative effort and financial costs outweighed potential benefits. By being agile and flexible on the development of policy, COVAX was able to mitigate some of the risks and challenges of operating at a time of supply constraints and demand uncertainty, and limit the wastage of doses.
Key results in 2021
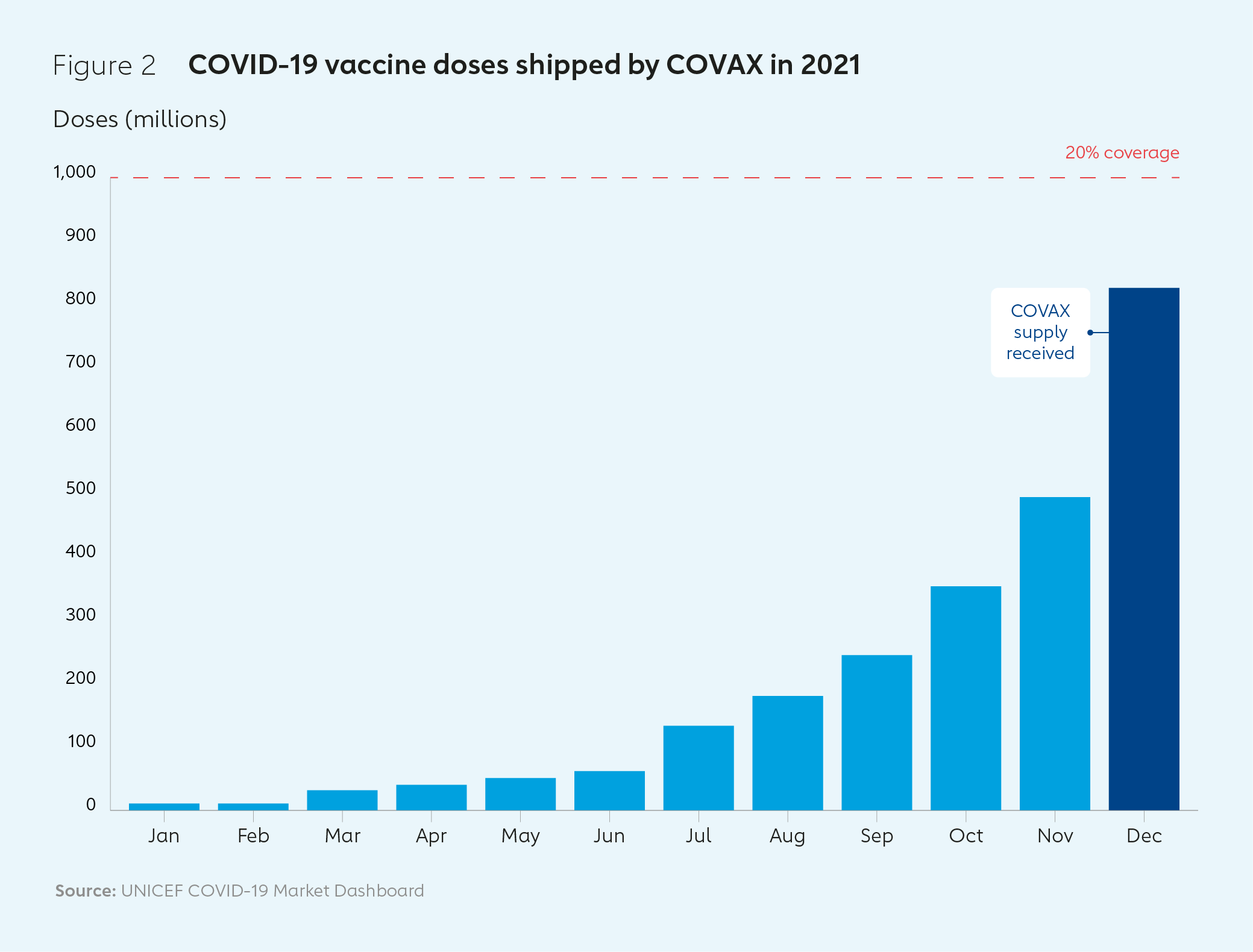
COVAX’s first international delivery of COVID-19 vaccines arrived in Ghana in February 2021. COVAX shipped almost 1 billion doses to 144 countries by the end of the year, with the billionth dose landing on a plane that touched down in in Kigali, Rwanda, on 15 January 2022. During 2021, 830 million doses were delivered to the 92 low- and middle-income Gavi COVAX AMC countries (AMC92) – 13% short of COVAX’s target of 950 million, despite well-documented challenges. The proportion of the population who had received a full primary series of two doses in AMC91 countries (AMC92 minus India) grew from 3% in June 2021 to 27% by December of that year.
3. Accelerating delivery
By January 2022, more than 12 billion doses of COVID-19 vaccines had been delivered worldwide from all sources. There was, however, a large and growing vaccine equity gap, as high-income and upper middle-income countries together accounted for 8 billion of these, while low-income countries (LICs) had only received 0.2 billion doses.3 At that time, only 13% of people in LICs had been fully vaccinated with a primary series of two doses, compared to 60% of the global population.
The causes of low vaccination rates varied from country to country. Funding issues related mainly to operational costs; payments and incentives for health care workers; training costs; transport; and logistics. In some places, political leadership was lacking, sometimes because COVID-19 had to be considered against a range of other competing priorities. There were also operational challenges that impacted countries’ abilities to absorb doses within short timeframes, including transport problems; lack of trained health care workers; and demand issues, such as a lack of information, vaccine hesitancy and COVID-19 misinformation. Funding issues related mainly to operational costs, payments and incentives for health care workers, training costs, transport and logistics.
Gavi, WHO and UNICEF created the COVID-19 Vaccine Delivery Partnership (CoVDP) in January 2022 to provide focused support to the 34 countries that were at or below 10% primary series coverage at this time. CoVDP focused especially on country engagement, demand planning, providing operational funding, delivery coordination, and reaching high-priority groups: health care and other frontline workers, older adults and those living with comorbidities.
In parallel, it was clear that after two years of emergency response and the impact of the pandemic on routine immunisation programmes, countries needed support integrating COVID-19 vaccination into regular health service delivery systems. In July 2022, Gavi launched another CDS funding round with an additional US$ 667 million and an expanded set of objectives:
- support acceleration of vaccination of high- and highest-risk populations, as defined by WHO Strategic Advisory Group of Experts on Immunization (SAGE);
- support rapid delivery of scale-up to reach country targets for adult vaccination; and
- support integration of COVID-19 vaccination and routine immunisation to achieve sustainable benefits.
The new CDS funds were also used to address some identified gaps in vaccine delivery and areas requiring strengthened engagement. For example, US$ 25 million was earmarked for direct support to civil society organisations to help unblock critical delivery, access and uptake bottlenecks, and reach under-served and unserved populations including high-risk groups and those in humanitarian settings. Another US$ 30 million was reserved for emergency funding to be jointly managed with the CoVDP for immediate needs in the 34 low-coverage countries. In total, this brought Gavi funding to support COVID-19 vaccine delivery across 2020–2022 to more than US$ 1.5 billion.
Funding through CDS provided important health system strengthening benefits in many countries. Of those that reported impact of CDS funding by October 2023, 71% of lower-income countries supported by COVAX reported strengthening of their cold chain infrastructure, while 76% were able to make advances in the digitisation of health data. A further 62% of countries reported integrating COVID-19 vaccination into their routine immunisation programmes.
Targeted investments to support COVID-19 vaccine deployment and scale-up
Management surge support
When COVID-19 was characterised as a pandemic in March 2020, countries already had routine immunisation systems in place. However, these were predominantly targeted at infants or adolescents, and many countries did not have adult vaccination programmes. COVID-19 also meant countries would need to deliver many times more vaccine doses than normal, faster than normal, and with little lead time to prepare.
In June 2021, Gavi agreed to provide funding to help strengthen countries’ programme management capacities and reinforce existing systems managing COVAX delivery, such as through Expanded Programme on Immunization (EPI) teams. In response, 25 countries requested assistance, mainly for programme management, data analytics and supply chain support. Almost 60% of management surge support (MSS) requests highlighted the need to reduce the impacts of COVID-19 vaccination on routine immunisation. The support was implemented from late 2021 and throughout 2022. For example, Gavi provided support to UNICEF for the recruitment of vaccine management specialists in 40 countries to help governments regularly review data on vaccine stocks, expiry dates, wastage and operational challenges. These specialists also supported countries in developing and implementing plans to help them avoid vaccine wastage.
This approach scaled up the processes and partnerships established during Gavi’s 2016–2020 strategic period (Gavi 4.0), which emphasised strengthening in-country leadership, management and coordination (LMC) to improve the sustainability of immunisation programmes.
Chad, for example, received MSS funding to strengthen governance of its COVID-19 national coordination committee and its EPI team. Funds were directed towards supporting the Ministry of Health in evidence‑based decision-making and providing management and leadership skills training for EPI staff to sustain routine immunisation and help increase COVID-19 vaccine uptake.
Nigeria’s MSS grant was the largest in the programme. It was used to strengthen COVID-19 vaccine delivery and management, and coordination with routine immunisation programmes. Funds were distributed to 10 separate health administrations of Nigeria’s 36 states.
Afghanistan’s MSS funds went towards, among other things, improving the consistency and quality of COVID-19 vaccine delivery, and enhancing coordination with routine immunisation activities. Funds were used to gather more key data; produce data visualisations and analytics; and better target resources through improved, evidence-based decision-making.
Innovation
Data from the Medicine Quality Research Group at the Centre for Tropical Medicine and Global Health, University of Oxford, identified instances of COVID-19 vaccine quality issues, such as falsified and degraded doses.4 Gavi, UNICEF and other partners collaborated to support COVID-19 vaccine introduction and scale-up by enabling vaccine authenticity verification and traceability. In 2020, Gavi provided US$ 5 million to kick-start development and testing of the Traceability and Verification System (TRVST), a tool designed to allow countries to verify the authenticity of health products, and to track and trace them through supply chains. TRVST has been successfully piloted in Nigeria and Rwanda, with Malawi, United Republic of Tanzania, Liberia and Nepal also signed up – and 12 other countries having expressed an interest in using it. Work is also underway on a second version that allows easier integration with existing country systems.
Working with civil society organisations
Community engagement, led by civil society organisations, was critical in raising awareness and increasing vaccine demand in many countries. Government support for community-led organisations and initiatives proved invaluable in many low-coverage countries that made significant progress in vaccine delivery during 2022. In addition, the US$ 25 million of CDS funding directed at leveraging the expertise, experience and networks of civil society organisations in July 2022 helped amplify the critical roles played by community groups in a wide variety of ways.
In Sudan, for example, Save the Children and their network of local partners worked with the government to bolster COVID-19 vaccine delivery efforts in two states with low coverage rates. A task force was established at the Ministry of Health in Chad to attract the involvement of local civil society organisations as partners to support government efforts to drive vaccine delivery. Community engagement was also key to Nicaragua’s remarkable achievement of increasing the proportion of its population that was fully vaccinated from 40% in January 2022 to nearly 90% by the end of the year. In the United Republic of Tanzania, activism played a major role in ensuring the success of COVID-19 vaccination after a slow start. Indeed, many of the countries that saw the most significant improvements in coverage relied on civil society and community engagement as keys to success.
Humanitarian delivery
By the end of 2022, of the 31 countries classified as having humanitarian emergency response plans, 26 were AMC participants. More than 250 million people are estimated to have needed humanitarian assistance across these countries. As of the end of 2022, half of all COVID-19 vaccine doses delivered in those 31 countries (411 million doses) were provided to their governments through COVAX, making it the largest source of vaccines in humanitarian contexts.
In 2020 and early 2021, Gavi collaborated with the United Nations’ Inter-Agency Standing Committee (IASC) – a forum for UN agencies involved in humanitarian work whose membership includes WHO and UNICEF – to design the COVAX Humanitarian Buffer, a safety net of last resort to ensure access to COVID-19 vaccines for the most high-risk and vulnerable populations: those in humanitarian settings, including refugees, migrants, asylum seekers, stateless people and other vulnerable groups. In March 2021, the Gavi Board agreed that up to 5% of doses procured through the COVAX Facility – up to 100 million doses by the end of 2021 – should be reserved for these groups via the Buffer.
As a first-of-its-kind pandemic innovation, the Buffer faced several hurdles, and its ultimate impact was limited. While it played a role in highlighting the needs of the most vulnerable in the global response, the Buffer ran into a range of challenges delivering novel products, particularly outside the normal state-based systems. Legal, regulatory, importation and political complexities were the norm as partners sought to work with non-governmental organisations to reach territories with little government presence, as well as those affected by issues relating to cross-border and sovereignty disputes. Of nine applications for doses, six were approved, with only two being finalised.
Gavi also needed to negotiate with manufacturers to obtain waivers for the indemnification for product liability they required from countries, and while the majority of manufacturers supplying COVAX ultimately agreed, this cost valuable time. Even then, these waivers did not cover all the potential liabilities involved in delivering vaccines to these settings, and these could not be the responsibility of non-governmental organisations. Resolving these issues required extensive negotiations between Gavi and other delivery partners, with Gavi ultimately having the highest risk capacity of the Buffer partner organisations.
Nonetheless, in November 2021, some 1.6 million COVID-19 vaccine doses were delivered to Iran through the Humanitarian Buffer, for vaccination of Afghan refugees after the return of the Taliban to power. This resulted in around 1.1 million individuals receiving vaccines, of which 500,000 received two doses – overcoming the challenges related to populations of concern living in hard-to-reach areas, such as low awareness about the importance of vaccination and social stress caused by asylum and migration.
In March 2022, some 840,000 doses were delivered, also through the Buffer, to support vaccination of refugees in Uganda. The resulting vaccination programme included important social mobilisation efforts and benefitted from increased efficiencies through the use of improved digital data tools.
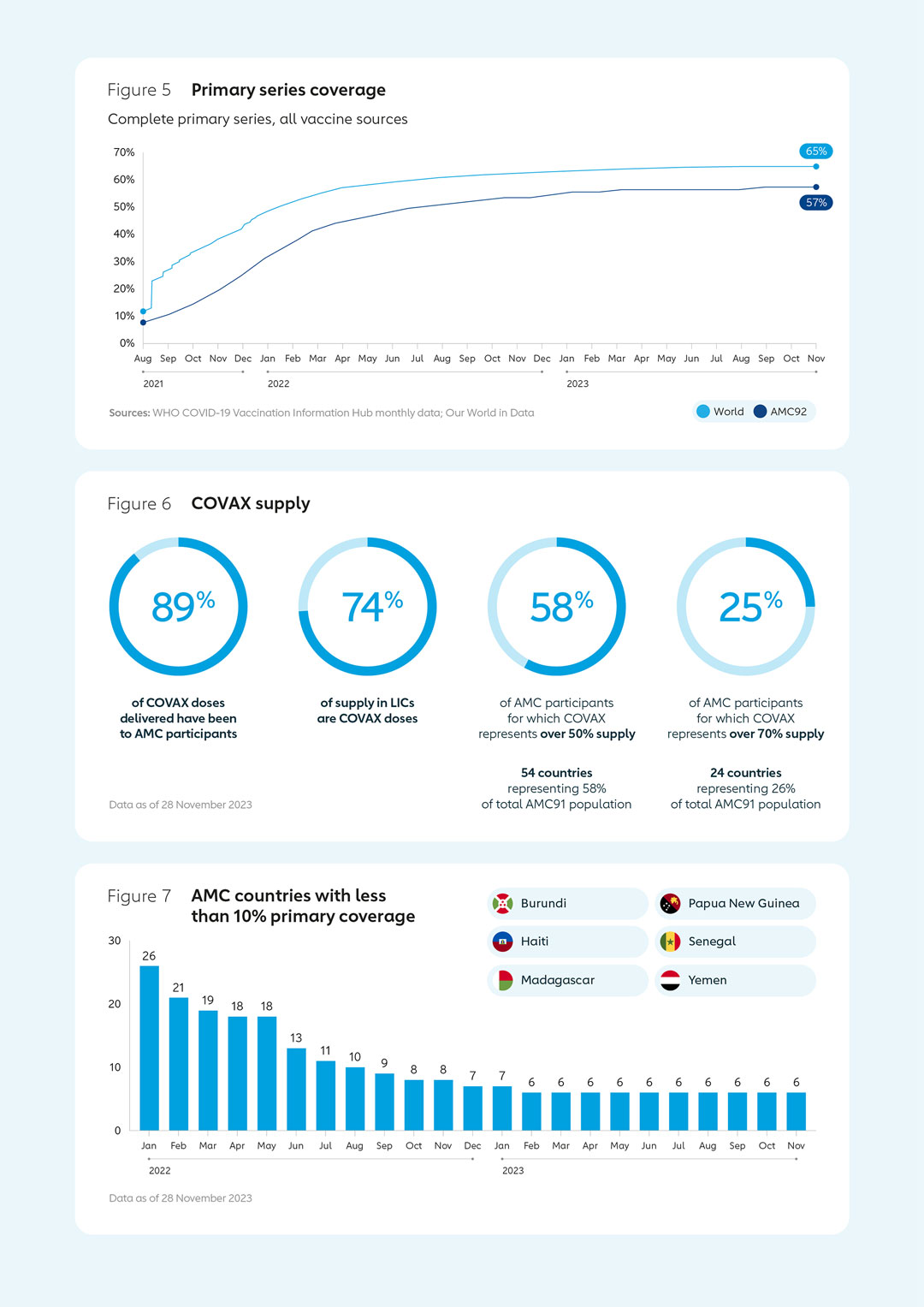
Key results in 2022COVAX delivered around 850 million COVID-19 vaccine doses in 2022. Complete primary series coverage reached 53% across AMC countries in December 2022, up from 31% in December 2021. With targeted support, the number of AMC countries below 10% full coverage dropped from 34 to 7 during the same period. COVID-19 vaccine boosting was introduced by 82% of AMC countries. Strong progress was also made in reaching high-priority groups.  | 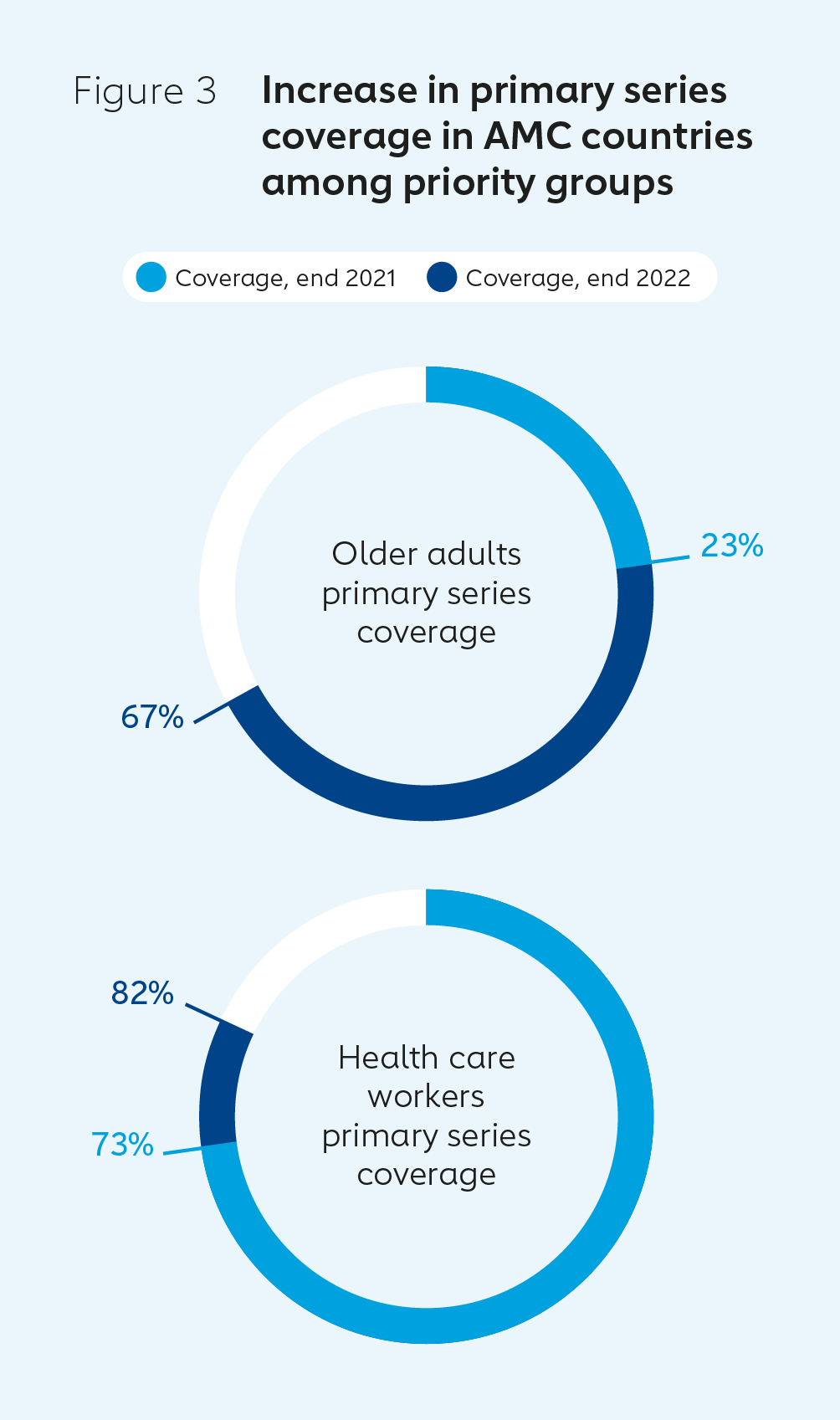 |
Increasing vaccine coverage and strengthening health systems in SomaliaIn January 2022, the COVID-19 vaccine coverage rate in Somalia was just 5%. Following initial difficulties in accessing and delivering doses, a series of campaigns between August and December 2022 enabled 90% of the targeted high-risk and older adult population to be reached. CDS funding was used, for example, to help scale up the country’s cold chain capacity. By May 2023, Somalia’s COVID-19 vaccine coverage rate had reached 41%. Many of the investments made to address the COVID-19 pandemic will have broader, long-term benefits for the country’s health system and people. These include: building an online digital system and dashboard to allow real-time vaccination monitoring; training staff in risk communication and community engagement; and procuring and installing four UCC units and 25 solar direct drive (SDD) vaccine refrigerators. | 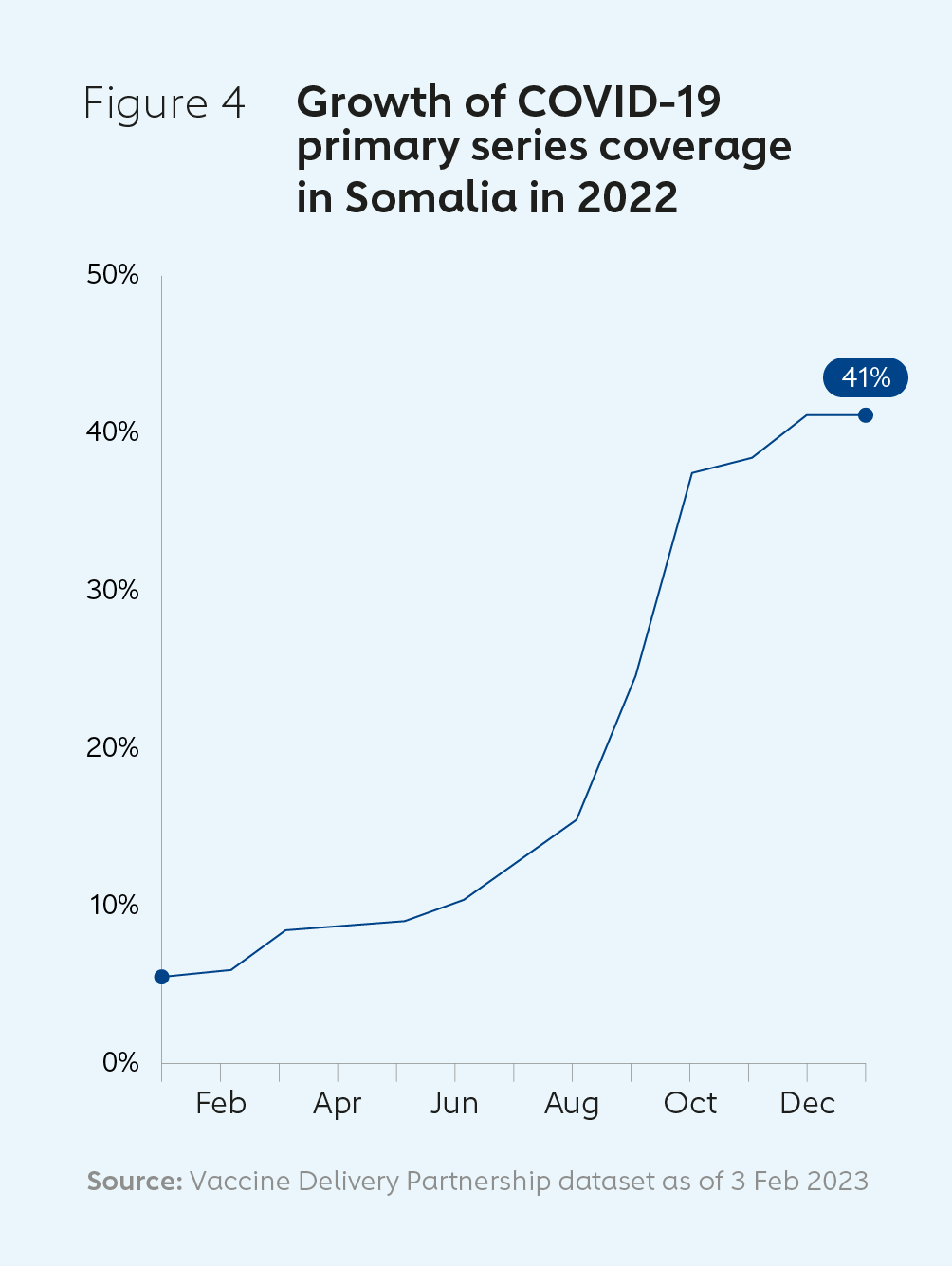 |
4. Delivering in 2023
In June 2022, the Gavi Board approved Gavi’s continued hosting of the COVAX Facility until the end of 2023. When asked at the end of 2022 how many COVID-19 vaccine doses they expected to need during 2023, the requests from AMC participant countries totalled 550 million doses. During the first three months of the year, demand fell well short of such projections, highlighting how the acute phase of the pandemic was continuing to recede. As of the 15 September deadline for dose requests from countries for allocation under COVAX for 2023, AMC countries had requested 120 million doses.
In March 2023, SAGE updated its guidance on COVID-19, including categorising population groups as low-, medium- and high-risk. The Group’s continued emphasis was on those at highest risk of death and severe disease – older adults, health care workers and people with immuno-compromising conditions. WHO announced the lifting of Public Health Emergency of International Concern (PHEIC) status for COVID-19 on 5 May 2023.
During the first half of 2023, the CoVDP continued to support the countries with the lowest COVID-19 coverage rates, with a focus on high-priority groups. As of June 2023, 6 countries had primary series vaccine coverage below 10%, compared to 34 in January 2022. Four of these – Madagascar, Yemen, Haiti and Burundi – were dealing with humanitarian situations. The Democratic Republic of the Congo achieved more than 10% coverage despite also having to deal with Ebola, cholera and measles outbreaks.
The CoVDP was always intended to be a time-limited mechanism to accelerate vaccine delivery in the countries with lowest uptake. In its final months, the Partnership supported the transition of COVID-19 vaccination programmes away from emergency response operations and into primary health care and routine immunisation services. The CoVDP was phased out in June 2023 as preparations were being made for the closure of the COVAX Facility at the end of the year, and integration of COVID-19 into standard operating models.
During 2023, Gavi continued to integrate COVAX functions into Secretariat and Alliance partners’ processes. CDS funding has continued to support COVID-19 vaccine delivery throughout the year. As of September 2023, 92% of the US$ 1.6 billion mobilised for CDS funding had been approved.
In June 2023, the Gavi Board approved a Gavi COVID-19 vaccine programme for 2024–2025. Its two core objectives are: to maximise health impact by continuing to support vaccine delivery for high-priority groups; and to continue to support health system strengthening and integration of COVID-19 vaccination into routine immunisation, primary health care and other health care services.
Gavi is continuing to work to ensure appropriate use of as-yet unspent funds, and to support countries in reallocating their approved CDS funding where needed to achieve the key objectives of the 2024–2025 programme. An additional US$ 20 million contingency fund has been made available to support countries that need extra support for COVID-19 vaccine delivery during 2024–2025.
5. Overall impact
Since its first deliveries in January 2021, COVAX has shipped some 1.98 billion doses of COVID-19 vaccines. Of these, 1.77 billion have been delivered free of cost to 87 AMC-supported countries.5
Modelling carried out by Imperial College London found that of an estimated 909,000 deaths averted by COVID-19 vaccination in African AMC countries by the end of 2022, 73% were averted by COVAX-supplied vaccines. Among low-income AMC countries, 75% of the 483,000 deaths avoided by use of COVAX-supplied vaccines came during the same period. Of the 13.3 million deaths avoided by COVID-19 vaccination in AMC countries worldwide by the end of 2022, 21% of these – or more than 2.7 million deaths – were averted by COVAX-supplied vaccines, or 34% if India is excluded from the figures. In May 2023, Gavi published a white paper on the preliminary assessment of COVAX’s impact in lower-income countries.
Before COVID-19, annual global production of all vaccines, including seasonal flu, was 3–5 billion.6 During 2021, some 12 billion vaccine doses were manufactured.7 Through 2021 and 2022, lower-income countries administered nearly 5 billion doses, several times more than the annual pre-pandemic volume.8 LICs were supported in their increased ability to deliver vaccine doses to their populations through Gavi CDS funding commitments totalling more than US$ 1.3 billion from June 2021 to July 2022. This helped drive improvements across the whole delivery journey, including supply chain, cold chain infrastructure, management support, social media monitoring, demand generation approaches for adults, health data digitisation and health care worker training.
However, many of the average percentages referenced above mask significant variations in the challenges faced by individual lower-income countries and their abilities to absorb large numbers of vaccine doses when they arrived. The complexity of understanding and addressing these challenges rapidly, and in ways that are suitably tailored to each national, subnational and community context, cannot be overstated. It is also important to celebrate and learn from success stories. Some countries, with support from COVAX, made spectacular progress in their coverage rates despite facing other major problems, including in some cases dealing with conflict and humanitarian crises.
Liberia, for example, ran a series of mass vaccination campaigns, with its Ministry of Health engaging with partners including religious, community and civil society leaders to communicate the importance of vaccination. As a result, coverage in the country rose from 20% at the start of 2022 to 74% in May 2023. Over the same period, the proportion of the Nicaraguan population fully vaccinated against COVID-19 rose from 40% to 92%. A shift in national policy in the United Republic of Tanzania kick-started efforts to counter vaccine disinformation and a national roll-out that successfully pushed coverage from 2% to 54% during 2022.9
6. Learning from COVID-19 to improve future vaccine delivery
Few, if any, countries and international organisations were well-prepared for COVID-19. Making decisions about how to respond effectively to a pandemic is inevitably difficult, especially during the early stages when its future evolution and impacts are hard to predict. COVID-19, however, has provided an opportunity for those thinking about how to better tackle future pandemics and other health emergencies. Gavi is among many organisations actively identifying ways in which it could have acted differently to mitigate the effects of COVID-19, especially on lower‑income and high-priority populations worldwide.
Roles and responsibilities
With other organisations taking the lead on vaccine delivery at the start of COVID-19, Gavi, as the secretariat of the Vaccine Alliance, originally planned to provide support for it only in exceptional circumstances. Partners were originally focused on their respective roles, while the Country Readiness and Delivery Global Working Group, established in April 2020, was designed to coordinate their efforts. There remained, however, some uncertainty about which organisations and agencies should coordinate different aspects of delivery, and it became increasingly apparent as 2020 progressed that there were gaps in delivery systems and mechanisms that threatened to prevent the successful roll-out of vaccines in lower-income countries. This led to COVAX taking on a growing role in supporting in-country delivery, and the establishment of the CoVDP, in January 2022.
Vaccine delivery is a complex process comprising many different elements, and almost nobody was ready for the challenges that confronted the world with the outbreak of COVID-19. It is nonetheless important that, going forward, international organisations – including Vaccine Alliance partners and other international health agencies, regional partners, civil society organisations and other stakeholders – collaborate and communicate with each other more effectively on the delivery of vaccines. The best way to achieve this is to create a coalition that works to ensure that the funding, systems, tools and people are all in place to deliver vaccines effectively and equitably from day one of any future outbreak, pandemic or other emergency. This requires that key roles, responsibilities, human resources requirements and surge capacity capabilities are discussed, clarified and agreed upon in advance of the health emergencies of tomorrow.
Organisations taking part in a future vaccine coalition should be allowed to play to their strengths. In 2022, countries immunised more than 68 million unique children with support from the Vaccine Alliance, bringing the total number of children to have received routine immunisation to more than 1 billion, or one eighth of humanity, since 2000. This track record, in addition to the resilience of the Alliance model for harnessing the expertise and political advocacy platforms of partners, other organisations and wider networks, means the Alliance should be well placed to play a central role in establishing such a coalition.
Contingent, at-risk funding
The many months it took to raise the funds needed to finance COVAX created delays that impacted those in lower-income countries hardest. Much of the resulting inequality in global access to COVID-19 vaccines could have been avoided had there been mechanisms in place to rapidly and automatically raise the money needed as soon as the pandemic was declared. In June 2021, the Gavi Board recognised that immunisation teams and health workers in many countries were in danger of being overstretched by imminent COVID-19 vaccine deliveries and agreed to provide delivery funding rapidly on a no regrets basis.
Discussions are ongoing between stakeholders about how best to avoid the funding delays experienced during the COVID-19 pandemic. Contingent financing mechanisms, which would see funds being released when trigger events such as a surge in illness or the spread of a new virus variant occur, would ensure more timely and equitable vaccine delivery support. Progress was made at Gavi’s Global Vaccine Impact Conference in June 2023, with the European Investment Bank (EIB) announcing an extension of its support to frontload grants to Gavi to boost routine and outbreak vaccination programmes, including a €1 billion financing facility potentially available for contingent grants, and U.S. International Development Finance Corporation (DFC) looking to expand its support for a range of critical immunisation efforts, including potential outbreak vaccines.
Funding pandemic responses brings with it risks of money being misused, duplicating financing from other sources or wasted in other ways. While a balance is needed between avoiding such risks and rapid release of funds to mitigate harms, organisations including Gavi should adopt higher funding risk tolerances from an earlier stage to stem losses of life and health during future pandemics than they did during the early stages of COVID-19.
Country engagement and advocacy
COVID-19 highlighted the need for strong engagement and dialogue with countries to understand their vaccine delivery challenges and provide effective support to address them. Communications with countries around aspects of delivery were sub-optimal during the early stages of the pandemic due to the lack of clarity concerning the roles and responsibilities of different organisations. Initially, too many organisations and teams within them were operating in silos, resulting in both duplication of effort and gaps in the provision of resources. This was also the case for communications from funders to countries on priorities and what support was on offer. It reinforced the benefits of engaging with political and financial leaders to advocate for the potentially key role of vaccination in avoiding deaths, disease and economic hardship.
In some places, the spread of misinformation about COVID-19 and vaccines led people to make decisions that cost many people their lives, health or livelihoods. Neither countries nor global health agencies including Vaccine Alliance partners had the systems and tools in place to counter this. Future preparedness planning must include putting in place better monitoring for mis- and disinformation, as well as broader initiatives to engage communities and build trust, such as using influential role models to build confidence.
High-level political engagement, beyond national health departments, can also play a role by supporting the removal of financial barriers to vaccine importation such as import taxes, and the creation of new multisectoral collaborations such as between ministries of health and ministries of education to support delivery to new populations. Support for countries can be more effective in future if organisations are able to work around single-country response plans from earlier on in any health emergency. Outcomes are also likely to be improved if countries can interact with one core organisation or partnership for all their immunisation needs.
Protecting routine immunisation
As Gavi highlighted early in the pandemic, the need to rapidly establish systems to roll out COVID-19 vaccination brought with it a risk of undermining vital routine immunisation programmes against deadly diseases such as measles, polio, yellow fever and diphtheria. Health care workers and their managers only have so much capacity. Research has shown that there were significant declines in routine immunisation coverage worldwide during the COVID-19 pandemic.
Plans to provide in-country surge capacity for health care workers, managers and other frontline employees are needed to enable effective responses to future pandemics. Going forward, Gavi and others must better plan for and take greater account of the need to protect routine immunisation programmes during health emergencies.
Targeting support
COVID-19 highlighted how, during health emergencies, countries in different circumstances need different types and levels of support. When procuring vaccines from manufacturers, for example, it was beneficial to deal with manufacturers on behalf of a large pool of countries across a wide range of income levels. Towards the end of 2021, however, the growing realisation that lower-income countries were being left behind in the race to secure vaccines provided the impetus to establish CoVDP and to prioritise those countries below 10% vaccine coverage for concerted delivery support.
The conditions and challenges in individual countries – from vaccine demand and health care worker training, to vaccine absorption capacity and resource levels – can vary significantly. Factors that affect vaccine supply and demand can change quickly during a pandemic. In the case of COVID-19, vaccination targets relating to priority groups and the proportions of the population to reach evolved over time. Epidemiological changes, as well as shifting political, cultural and socioeconomic realities, meant repeated adaptations were needed to the nature of support provided to countries.
Delivery support and decision-making must be context-specific and flexible in the face of changing events and circumstances. Greater understanding of the need for differentiated support is needed, as is earlier planning around the provision of additional delivery support to lower-income countries with the weakest health care systems and the targeting of resources where they will be most effective during the international health emergencies of tomorrow.
Reaching humanitarian settings
A great deal of time was needed to establish the mechanisms and policies needed to help support delivery of vaccines to those in humanitarian settings during the COVID-19 pandemic, not least in securing the required vaccine indemnification and liability waivers, and broader legal agreements. The COVAX experience has provided the foundations for future legal considerations associated with supply of vaccines during pandemics in humanitarian and emergency settings. COVID-19 has also highlighted that proactive communications and targeted outreach may be needed to generate demand and boost confidence in vaccines during future humanitarian engagements. A principled and conflict-sensitive method of ensuring distribution of vaccines across borders, which respects national or international sanctions, should be in place in the very early stages of any pandemic to avoid delivery delays.
Further learning
Gavi has commissioned an independent, multi-stage evaluation of what worked well and what worked less well in the design and implementation of the COVAX Facility and COVAX AMC. Phase 1 of this evaluation, covering the period from the launch of COVAX in 2020 to the end of 2021, was published March 2023. Work on phase 2, covering 2022–2023 and due to begin in 2024, which includes a joint cross-partner evaluation of COVAX delivery, will play a key role in highlighting further valuable learnings relating to the challenges and successes of COVAX partners in the delivery of vaccines, with a particular focus on the efforts of Gavi, WHO and UNICEF, during the COVID-19 pandemic.
7. Conclusion
Gavi has worked for more than 20 years to strengthen not just immunisation programmes, but the health systems that run them as part of its commitment to saving lives and protecting health through increased, equitable and sustainable use of vaccines. The ways it has done so are many and varied. These include improvements in health and stock management information systems; the use of data; supply chains; cold chain and other infrastructure; leadership and management capabilities; health care worker training; and public information campaigns.
The health care systems of countries of all incomes were poorly prepared for COVID-19. There is, however, no doubt that the health care infrastructures in many LICs were in a better state at the start of the pandemic than they would have been without the Vaccine Alliance’s investments in HSS prior to 2020, such as through the support of sub-national and remote health care facilities, as well as digital health information systems used for COVID-19 surveillance and to track vaccination. These investments laid some of the foundations that enabled the 57 lower-income countries supported by Gavi in the 2021–2025 strategic period to almost triple the number of vaccine doses they were able to administer: from 975 million in 2020 to 2.7 billion in 2022, including routine immunisation and other vaccinations (but excluding polio vaccines, which are administered orally and do not need trained vaccinators).
As COVID-19 spread between countries and case numbers grew rapidly in the first half of 2020, the pandemic threatened to overwhelm health systems worldwide, putting at risk many gains made during the previous two decades. Gavi and COVAX provided support for lower-income countries across the journey that vaccines make to get into the arms of the individuals who need them. More than 2.7 million deaths were averted by COVAX-supplied vaccines.
The investments made by Gavi and COVAX to support vaccine delivery have provided opportunities for beneficial long-term legacies. Public engagement and communications work helped make the political, economic and moral cases for ensuring everyone has access to immunisation and effective health care. Investments in immunisation programmes and health system capacity, such as in public information campaigns, for example, will benefit health systems and the populations they serve for years to come.
The additional capacity built to identify and reach hard-to-reach populations and adults with COVID-19 vaccines can be deployed to deliver other vaccines, including those that already exist and those of the future. Investments made in new vaccine technologies, ways to scale them and other innovations developed in response to COVID-19, are already being applied to other conditions. Gavi is applying the lessons of COVID-19, both in its planning for future pandemics, and to improve the ways it delivers routine and other forms of immunisation.
COVID-19 would have caused many more deaths, higher numbers of cases of illness and greater economic disruption without the investments made in strengthening health care systems in lower-income countries prior to the pandemic. The vaccine delivery support provided for COVID-19 not only mitigated the pandemic’s impacts but will deliver significant long-term health benefits for many years. Gavi stands ready to work with its partners to defend the gains of the last 20 years, maximise the health impacts of the investments made during COVID-19, and to save many, many more lives through the delivery of vaccines in lower-income countries in the years to come.
References
- https://covid19.who.int/
- https://www.economist.com/graphic-detail/coronavirus-excess-deaths-estimates
- https://www.unicef.org/supply/COVID-19-market-dashboard
- https://www.iddo.org/document/medical-product-quality-report-COVID-19-vaccine-issues-data-31-may-2021
- https://www.unicef.org/supply/covid-19-market-dashboard
- https://www.ifpma.org/insights/momentum-of-COVID-19-vaccine-manufacturing-scale-up-sufficient-for-step-change-in-distribution/
- https://www.ifpma.org/news/the-european-commission-and-innovative-biopharmaceutical-industries-to-explore-potential-initiatives-to-enhance-local-production-of-vaccines-and-treatments-in-africa/
- https://www.gavi.org/sites/default/files/covid/covax/COVAX-data-brief_17.pdf
- https://COVID19.who.int/table







