Vaccine profiles: hepatitis E
A vaccine against hepatitis E was recently deployed to quench an outbreak at a displaced persons’ camp in South Sudan. Could it soon be used more broadly?
- 11 December 2023
- 7 min read
- by Linda Geddes
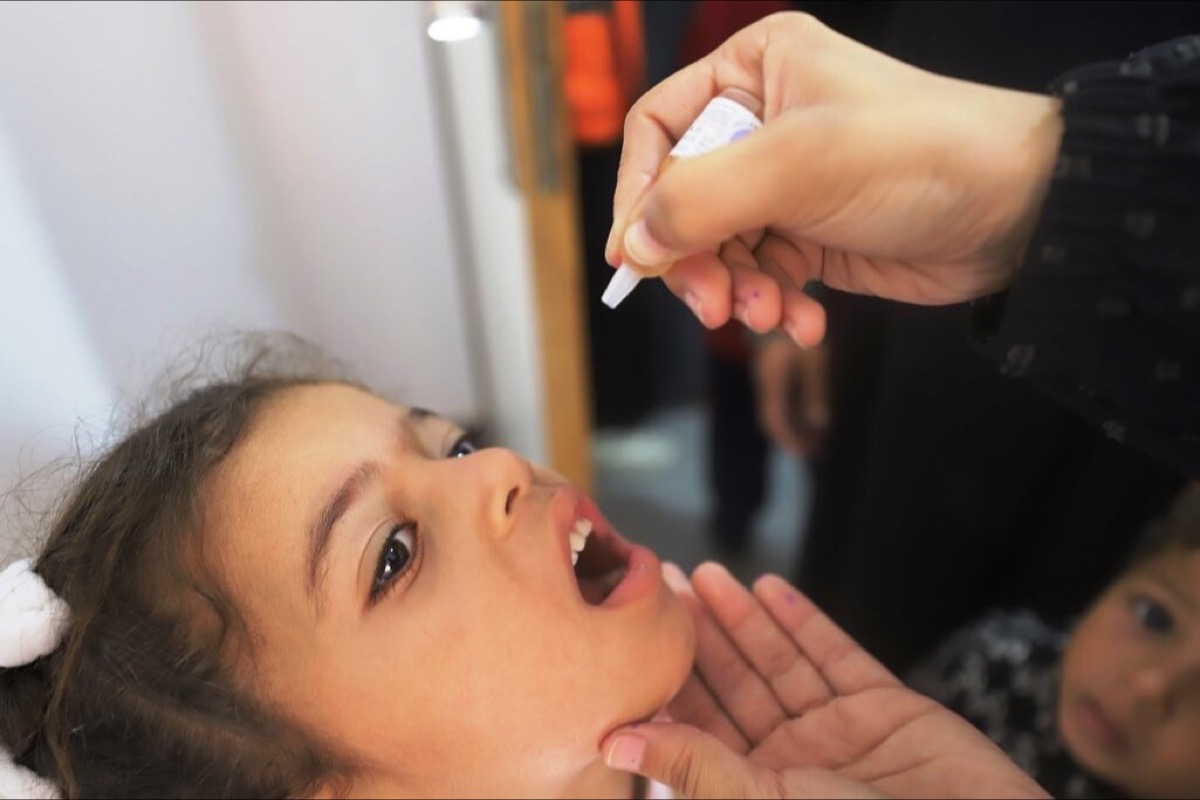
In 2021, heavy rainstorms flooded shelters and latrines at the Bentiu displaced persons camp in South Sudan, making the already deplorable living conditions even worse. As children played in sewage-contaminated floodwaters, and families resorted to eating waterlilies when aid supplies struggled to get through, it wasn't long before people living there started to develop jaundice, fever, abdominal pain and other signs of the waterborne illness, hepatitis E.
Hepatitis E is a disease that affects the world’s most vulnerable populations, thriving in conditions where access to clean water, toilets and handwashing facilities is limited. Outbreaks among displaced populations, such as the Bentiu outbreak, are becoming more commonplace.
Bentiu is the largest displaced persons camp in the country, and hepatitis E outbreaks have been occurring there since 2015. But the 2021–22 outbreak was particularly severe, with 759 patients admitted to Médecins Sans Frontières' (MSF) Bentiu hospital between July 2021 and July 2022, 17 of whom died.
A decade earlier, the world's first hepatitis E vaccine had been approved by Chinese regulatory authorities, but despite a World Health Organization (WHO) recommendation that it be considered for use in outbreak responses, it had never been used outside China. But with conditions in Bentiu worsening and hepatitis E cases rising, South Sudan's Ministry of Health took the unprecedented step of asking MSF to incorporate the vaccine into its outbreak response.
Hepatitis E is a disease that affects the world's most vulnerable populations, thriving in conditions where access to clean water, toilets and handwashing facilities is limited. Outbreaks among displaced populations, such as the Bentiu outbreak, are becoming more commonplace.
Although an effective vaccine exists, a paucity of data from populations outside China, lack of experience with the vaccine, limited availability, and the absence of WHO-prequalification – an internationally-recognised standard attesting to the safety, quality and efficacy of vaccines and other medical products – has limited its use until now.
Symptoms can include fatigue, poor appetite, stomach pain, nausea, and jaundice and, while many people have no symptoms and recover from the disease without any complications, some develop acute liver failure, which can be fatal.
However, thanks to recent trials and the first ever hepatitis E mass vaccination campaign in South Sudan in 2022, these data gaps are starting to be filled, raising hopes that hepatitis E outbreaks could soon be consigned to history.
What is hepatitis E?
Hepatitis E is a liver infection caused by the hepatitis E virus (HEV), which is found in the stool of infected individuals. It is mostly spread through drinking water contaminated with their faeces, although infections also occasionally occur through eating raw or undercooked pork, venison, wild boar or shellfish.
Symptoms can include fatigue, poor appetite, stomach pain, nausea, and jaundice and, while many people have no symptoms and recover from the disease without any complications, some develop acute liver failure, which can be fatal. Pregnant women – particularly those in the second or third trimester – are at greatest risk, with up to 25% of those infected during their third trimester dying from the disease. They are also at increased risk of miscarriage and stillbirth.
Hepatitis E can also cause chronic infections in immunocompromised patients, such as those with HIV or who have received an organ transplant, resulting in liver damage and scarring (cirrhosis).
There is no specific treatment, so the most effective approach is trying to prevent infections through access to clean water, sanitation and handwashing facilities.
According to WHO, approximately one third of the global population has been exposed to hepatitis E virus, and 35 million people are infected annually, resulting in 70,000 deaths – mostly in poor and vulnerable populations with limited access to clean water.
There are approximately four to five outbreaks reported in Gavi-supported countries each year, and these outbreaks are becoming more and more serious. The greatest burden of disease is in India and sub-Saharan Africa, but outbreaks are increasingly occurring in war zones and camps for refugees or internally displaced populations, where sanitation and safe water cannot be guaranteed.
Vaccine development
The hepatitis E virus was first identified in the 1980s, with further research revealing at least four different types. Genotypes 1 and 2 only infect humans and are responsible for ehpatitis E epidemics in developing countries, while genotypes 3 and 4 also circulate in animals and are mainly responsible for sporadic human cases, which have been increasingly reported in developed countries since the late 1990s.
Because there is no specific treatment for hepatitis E, the development of an effective vaccine is crucial to help prevent and control this disease.
Because there is no specific treatment for hepatitis E, the development of an effective vaccine is crucial to help prevent and control this disease. However, scientists have struggled to grow the virus in the laboratory, making it impossible to develop traditional live or inactivated vaccines. Instead, efforts have focused on the manufacturing the structural, capsid protein that encloses the virus's genetic material.
Clinical trials of several vaccine candidates were conducted in in the 2000s, including a candidate developed by GSK in collaboration with the US military. Although a proof-of-concept trial in 2,000 soldiers suggested that preventing hepatitis E through vaccination was feasible, GSK could not identify public-sector partners who were willing to invest in further development of the vaccine, and the project was shelved.
Have you read?
Meanwhile, Chinese scientists had been developing their own vaccine candidate. Hepatitis E is endemic in China, with nine outbreaks recorded between 1982 and 1991, the largest of which resulted in some 120,000 cases and 707 deaths (414 of them in pregnant women) between 1986 and 1988.
Prompted by such outbreaks, Chinese scientists set out to develop a vaccine based on pieces of capsid protein from the genotype 1 hepatitis E virus that self-assemble into virus-like particles (VLP) – structures that physically resembles the virus, but don't contain any genetic material so are unable to infect cells or reproduce. VLP-based vaccines have also been developed and licensed against other viruses, including human papillovirus and hepatitis B.
Licensed vaccine
The hepatitis E vaccine HEV p239 (Hecolin®) was developed by Xiamen Innovax Biotech and approved by the Chinese Food and Drug Administration in 2011. A clinical trial involving 112,604 healthy 16- to 65-year-olds suggested an efficacy of 100% in preventing clinical hepatitis E among those who had received three doses. A longer-term evaluation suggested an efficacy of 87% four years after receiving a third dose.
The Hecolin vaccine has been available in China since October 2012, where it is recommended for people aged 16 years and above who are at high risk of hepatitis E infection. This includes those involved in animal husbandry, food handlers, students, members of the armed forces, women of childbearing age, and travellers to endemic areas.
In 2015, WHO recommended that national authorities consider using the Hecolin vaccine to control outbreaks, but its wider introduction has been hindered by a lack of clinical data from countries in Africa, Southeast Asia and South Asia countries, where hepatitis E is more prevalent and where genotypes I and II are the dominant types. The dominant genotype in China is genotype 4, with some genotype 1.
Also needed was safety and efficacy data from children, elderly patients, those with chronic liver diseases or immune disorders and, most importantly, pregnant women.
In early 2021 Médecins Sans Frontières (MSF) established a stockpile of 50,000 doses of Hecolin for rapid epidemic responses. This was mobilised for the first time the following year, to help bring the outbreak at Bentiu internally displaced persons camp under control.
Between March and October 2022, around 27,000 16- to 65-year-olds living in the camp, including pregnant women, were offered three doses of the vaccine, with 113% of the initial target of 26,848 people reached in the third round.
Hepatitis E is endemic in China, with nine outbreaks recorded between 1982 and 1991, the largest of which resulted in some 120,000 cases and 707 deaths (414 of them in pregnant women) between 1986 and 1988.
The campaign highlighted several challenges associated with the transport, storage, and waste management of the vaccine, which could provide valuable lessons for future vaccination campaigns. Ongoing studies are also generating further data on the vaccine's effectiveness and safety in the context of this epidemic response. Such observational studies could also provide valuable information about the level of protection afforded by just one or two doses of the vaccine – as delivering the recommended three doses is costly and challenging in an outbreak setting, particularly among displaced populations with limited health care infrastructure.
Other recent trials have demonstrated that Hecolin is safe and generates an immune response among those aged over 65 years, and people who are also infected with hepatitis B, while the results of a study in people with chronic liver disease are expected to be published soon.
While data in pregnant women and children is still needed, such studies are gradually filling the gaps that are holding back the wider rollout of this vaccine. Given the public health threat posed by hepatitis E, this data couldn't come soon enough.
More from Linda Geddes
Recommended for you