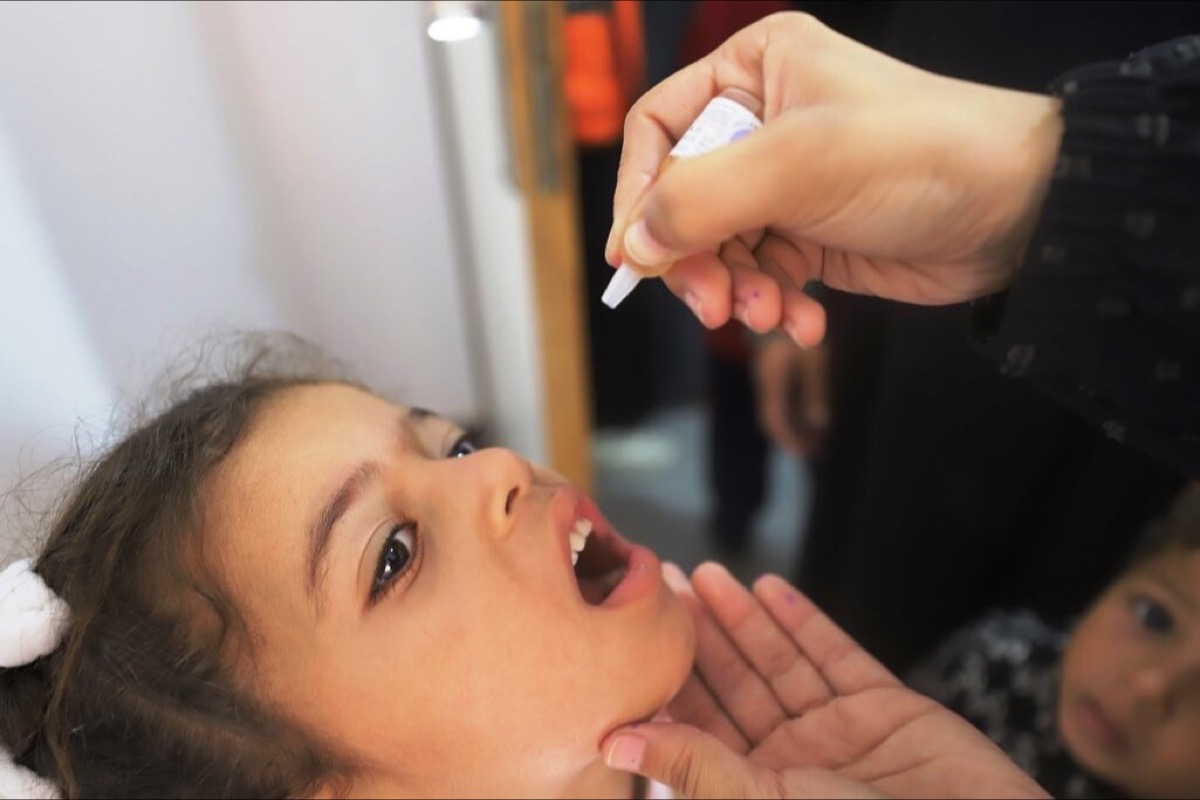
Six things you need to know about the latest tool against polio
WHO prequalification of the novel oral polio vaccine type 2 (nOPV2) should make it easier for countries to tackle outbreaks.
- 16 January 2024
- 5 min read
- by Linda Geddes
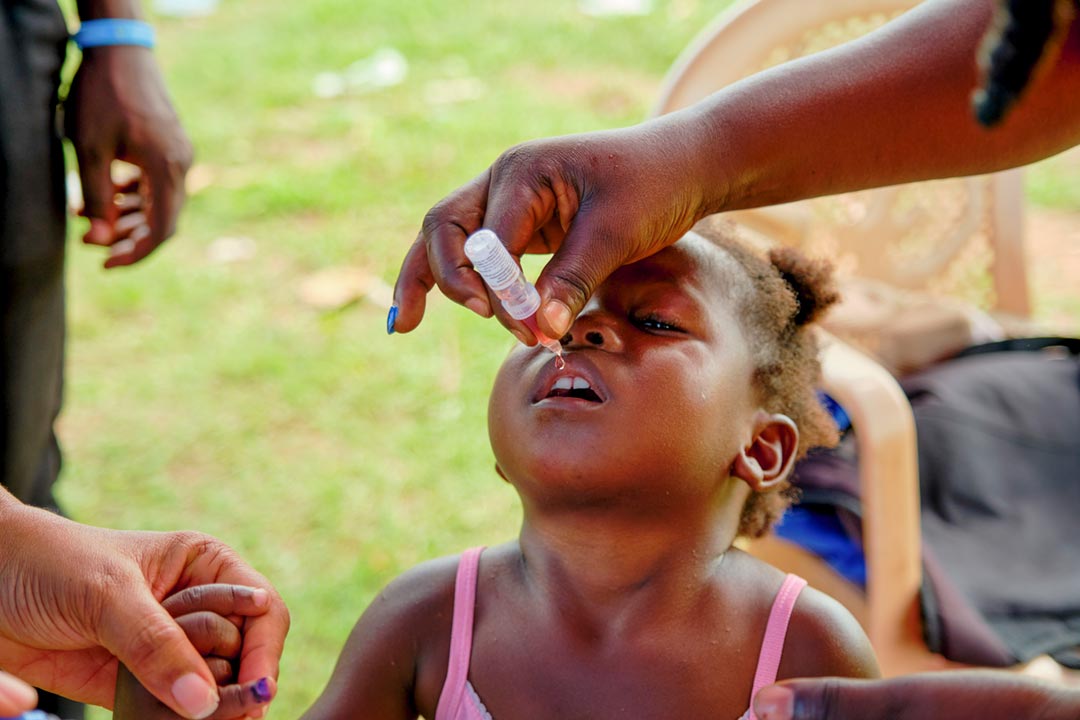
Polio is a tricky pathogen to crack. Although highly effective vaccines exist, a rare phenomenon known as variant poliovirus can occasionally occur when new strains of poliovirus are seeded in un- and under-immunised communities. These viruses, under the right conditions, can sometimes genetically revert, triggering outbreaks of paralysis-causing disease.
WHO prequalification is the final vetting process to ensure that the vaccines used in immunisation programmes are safe and effective. Now that nOPV2 has achieved this status, it should be easier for more countries to access and use nOPV2 in response to cVDPV2 outbreaks.
In 2021, a new polio vaccine was granted a World Health Organization (WHO) emergency use listing (EUL), to hasten its availability to help tackle such outbreaks.
Now this innovative vaccine – known as novel oral polio vaccine type 2 (nOPV2) – has been granted WHO prequalification, allowing for more streamlined regulatory approval in countries that need it. Here's everything you need to know about this latest tool in the global fight against polio.
1. Variant poliovirus must be addressed
Variant polio stems from the historic use of oral polio vaccine (OPV). Although OPV is highly effective, it contains live poliovirus strains that have been weakened. As they are live, they can replicate to prompt an immune response.
After children are vaccinated, some of the weakened viruses in the vaccine are excreted in their stools and transmitted to other people, enabling passive protection against polio. In communities where lots of people have been vaccinated against polio, such onward transmission quickly dies out. However, in communities with low vaccination coverage, the weakened virus may continue to circulate for many months, gradually accumulating mutations that enable it to cause paralysis.
Variant polio is rare, but as immunisation campaigns have eradicated two strains of wild poliovirus and banished the remaining strain from all but a few small pockets of the world, the circulation of variant poliovirus (cVPDV) in countries that had previously been declared polio-free is threatening to undermine the progress made to date. Unless all types of polioviruses are eradicated, polio will make a global comeback.
2. nOPV2 is more stable than previous oral polio vaccines
There are three strains of poliovirus (types 1, 2 and 3) and type 2 variant poliovirus outbreaks are the most prevalent. nOPV2 is a next-generation version of the traditional type 2 monovalent oral polio vaccine (mOPV2) that is used to respond to cVDPV2 outbreaks.
Have you read?
Clinical data and field use have demonstrated that nOPV2 is as safe to use and effective at stopping outbreaks as mOPV2; the difference is that the weakened type 2 poliovirus that the new vaccine contains has been modified to make it more genetically stable and significantly less likely to revert into a threatening form. Estimates suggest that nOPV2 is 80% less likely to seed new outbreaks of variant polio, increasing the chances of stopping these outbreaks for good.
3. A billion doses of nOPV2 have been used so far
nOPV2 was granted the WHO's Emergency Use Listing approval in March 2021. This mechanism enables the early and targeted use of yet-to-be licensed vaccines, diagnostic tests, and treatments in response to Public Health Emergencies of International Concern following careful analyses of existing clinical trial data. Since then, nOPV2 has been used in 35 countries that have been affected by cVDPV2 outbreaks, with close to a billion doses administered so far.
In Nigeria, where nearly half a billion doses have been given under the EUL, the vaccine has aided an 85% reduction in variant poliovirus cases since 2021.
4. WHO prequalification will help expand access to this vaccine.
Helpful as the Emergency Use Listing (EUL) has been, it meant that countries could only administer the vaccine after meeting strict readiness and monitoring requirements. While the polio programme supported countries who wished to use nOPV2 through the EUL verification process, those who were not yet verified relied on mOPV2, instead.
WHO prequalification is the final vetting process to ensure that the vaccines used in immunisation programmes are safe and effective. Now that nOPV2 has achieved this status, it should be easier for more countries to access and use nOPV2 in response to cVDPV2 outbreaks.
5. nOPV2 is only part of the solution.
nOPV2 is an important tool for polio eradication, especially in preventing and controlling cVDPV2 outbreaks, but is only one such tool. Ultimately, the plan is to phase out OPV altogether, and switch to using inactivated polio vaccine (IPV) – another type of vaccine that contains dead virus particles and has zero chance of seeding variant polioviruses. IPV is highly effective at protecting against paralysis;, but, unlike OPV, it doesn't prevent individuals from being infected asymptomatically with poliovirus and transmitting it to others.
Once polio has been eradicated from most continents and countries, the key to keeping it that way will be to maintain high levels of population immunity through routine childhood immunisation with IPV. Hexavalent, a 6-in-1 vaccine that includes IPV, will also be an important tool as we look toward a post-polio world.
6. Its approval could enable the expedited use of other new vaccines.
nOPV2 is the first vaccine to be WHO prequalified after EUL. This precedent could allow approvals for other new tools and innovations undergoing clinical development – including vaccines – for targeted responses to public health emergencies, with the possibility of broader use once full clinical data has been accrued.