Modelling the Manufacturing Process for COVID-19 Vaccines: Our Approach
Researchers across the world are working flat out to develop and manufacture a vaccine for COVID-19 that can end what has been the worst pandemic in at least a century.
- 1 September 2020
- 9 min read

Researchers across the world are working flat out to develop and manufacture a vaccine for COVID-19 that can end what has been the worst pandemic in at least a century. To better understand how long it will take to develop a vaccine and manufacture it at scale, CGD is teaming up with Ariadne Labs and others to conduct expert interviews and build a tool that will generate probabilistic estimates for the likelihood of success of different types of vaccine and how long it could take to develop a vaccine, and to model how long it will take to produce this vaccine at sufficient scale to meet global demand. This will build on the work of other tools which track the stages of development of different vaccines, to estimate the overall probability of success for vaccine candidates and the time taken to reach large-scale manufacturing targets. In this blog post, we focus on the manufacturing part of this process (which we are undertaking with Bryden Wood) and the steps we are taking to model the vaccine manufacturing timeline.
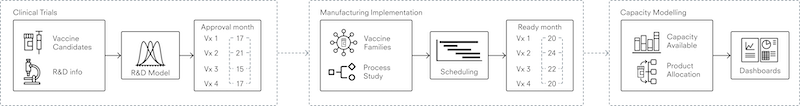
We are developing a system of interconnected models which represent global manufacturing capability from the start of clinical trials to secondary vaccine manufacture; that is, time from first human trials to finished product ready to be shipped. We will split the process of modelling vaccines research and development into three stages, as shown in figure 1: (i) modelling clinical trials until their approval, (ii) modelling the time it takes to scale up manufacturing, and (iii) modelling capacity to predict how long it will take to produce significant volumes of new vaccines.
Figure 1. Three stages of modelling the vaccine manufacturing process
Data collection
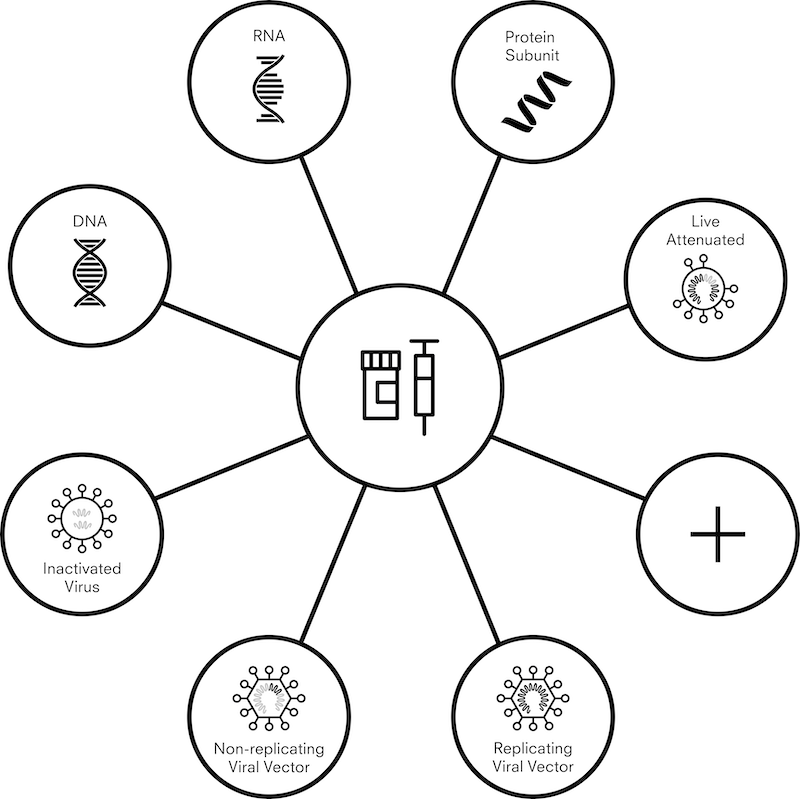
There are currently more than 200 vaccine candidates against COVID-19, and more than 300 manufacturing facilities across the world that are capable of producing vaccines. With so many moving parts – research continuing at a speed never before seen, and large amounts of the information being politically or commercially sensitive – data collection at this scale is not an exact science. To provide a practical and realistic framework for data collection, we are grouping vaccines into seven different “platforms.” These platforms range from more traditional and established methods – such as an inactivated viral vaccine, where virus particles with no ability to produce disease are used to stimulate an immune response – to newer technologies including DNA and RNA vaccines which produce an immune response without viral particles.
Figure 2. Vaccine candidate platforms
In light of this, a model must be able to function with uncertainty and provide useful indications within practical limitations. In order to achieve this we will us use ranges and distributions for plausible estimates and put these into a Monte Carlo simulation. This is a technique that estimates the probability of different outcomes by running multiple simulations with different estimates randomly chosen from a plausible range, and then aggregating the results.
Where factual inputs exist, these go straight into the model. We are then collecting ranges or plausible estimates from a number of sources, all of which is currently underway. These include:
- expert interviews
- interviews with development and manufacturing organizations
- a survey of research groups with a vaccine candidate
- independent research on vaccine candidates
We will use this information to estimate how many doses of vaccines can be produced in a given period. We will then combine this information with WHO- published estimates of doses required to cover priority groups, support enough coverage to protect healthcare systems, reduce COVID-19- related mortality, and ensure fair access to vaccines. As and when the WHO estimates of phased global demand are updated, we will update accordingly in our model.
Read more: COVAX / #VaccinesWork / About Gavi
R&D and clinical trials
To estimate how long it will take to manufacture enough vaccines, we must first know which vaccines need to be manufactured. To estimate the probability of success for different vaccine platforms, we interviewed 17 experts with expertise in vaccine development and vaccine manufacturing. We also collected information on funding for vaccine candidates, and on plausible timelines. We then put this into a Monte Carlo simulation model, which thousands of times, with each run, vaccines randomly succeed and fail in line with their perceived probability of success. While the results of an individual run will not tell us a lot about the vaccine portfolio, by aggregating the results we should get a sense of both timelines and numbers of vaccines that could be approved.
Manufacturing and implementation
This part of the model aims to estimate the time required after a vaccine has been approved before commercial manufacture can begin. This is an important, yet perhaps publicly underdiscussed, factor in the supply of any drug. A drug substance that has been manufactured for early stage clinical trials is most often at “R&D scale,” produced in facilities with lab or small-scale equipment to supply doses in the thousands. Scaling up manufacturing to commercial scale adds complexity. Therefore, any attempt at commercial manufacture in the order of millions or billions of doses needs to consider the steps to scale up the process and adapt it for manufacture at a given location; under normal circumstances, this could take a few years.
The transition from R&D to manufacturing is typically carried out by a large multifunctional team and includes process development activities, design and construction activities, and quality assurance/regulatory activities. We have broken this transition down and simplified it to form a series of steps for any vaccine. For each step, we assigned a probabilistic distribution of durations around a value based on experience. The distributions tend to be right skewed (meaning things take longer than planned) because, unfortunately, there are constraints that stop things being done in less than a certain time but many potential delays that are without constraints. Steps can overlap; in particular, scale-up and preparation of manufacturing facilities can start earlier than normal, “at risk” (of failure of the candidate).
Strict regulations to ensure that manufacturing processes and plants meet current good manufacturing practice (CGMP) guidelines also add time to the transition from R&D to commercial manufacturing. The plant and equipment must be qualified, and processes shown to be safe and effective and licensed by regulators from all the jurisdictions where the vaccine will be used. Data for the product, process, and plant must be submitted to regulators in every jurisdiction where the vaccine will be used, and regulators vary in their requirements and, often, their areas of focus. Regulators can ask for clarifications or improvements, sometimes requiring additional data to be generated or even modifications to plant or process. There are some intellectual property (IP) issues when companies are trying to scale up manufacturing using Contract Manufacturing Organisations, a particular risk for newer companies in the business with newer manufacturing platforms, who don’t always have an established process for how to deal with IP with suppliers of clinical material (this has already been a problem for two candidates). All of this can cause delay.
In addition to manufacturing capacity, such a large scale-up also requires sufficient quantities of auxiliary supplies, such as vials (or other primary containers), adjuvants, and in some cases, single-use bioreactors.
The overall sequence is well-understood, but there are many factors – such as type of vaccine and dosage form, manufacturing location, and supply markets – that affect how long each step takes. Through industry expert input, we are developing an initial model to represent each key stage of a vaccine’s journey from success in clinical trials to commercial manufacturing. The model incorporates possibilities that capacity already exists at sites, that existing capacity requires modification, or a new factory is required. This includes all steps of “qualification” – validation and testing processes that ensure equipment has been installed correctly and will perform as expected under real factory conditions. To account for variability, stages will be defined by ranges and distributions rather than single parameters.
Manufacturing capacity
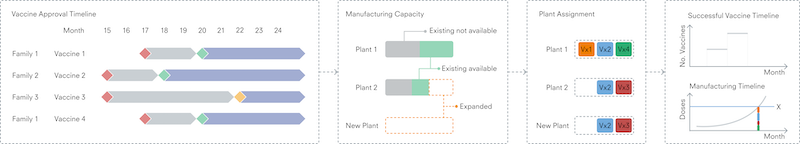
The primary output from the overall model is an understanding of when global vaccine demand – defined here by the WHO targets – may be met. It is intended to collect global manufacturing capability for primary (manufacture of the active ingredient, for instance virus or mRNA) and secondary (manufacture of the dose form, for instance a vial of a liquid solution of the active ingredient) manufacturing, which would be used to build a picture of currently available capacity and potential future capacity for the suite of vaccine platforms and technologies. CEPI recently published an extensive survey-based assessment of available capacity This data will inform our understanding of global manufacturing capability for different vaccine platforms, and be used in the manufacturing model to enable us to calculate the timelines.
Outputs from the R&D model are used a starting point, indicating when individual vaccine candidates may pass clinical trials – using a defined point in time as a reference point. Each successful vaccine will then pass through the manufacturing implementation model to determine a timeline for start of primary and secondary manufacture. Vaccines can then be allocated to available network capacity for the appropriate platform, computing the monthly dose production and cumulative dose count not only as a total, but also for each vaccine individually. This level of detail may be important given the possibility that different vaccines can be used for different populations (age ranges, for example) based on the level of immune response elicited. In comparing these production figures with the WHO targets, the time from the reference point to production milestones can be estimated.
Figure 3. Overview of the calculation stages and output of the manufacturing capacity model
Usage and next steps
We are currently developing and testing this model based on the functionality outlined above. Once we’ve completed preliminary data collection and building the model, it will be run for parameters as defined by data collection and expert input. Our aim is to be able to give initial estimates of timelines of when vaccines would be widely available to the patient population.
We hope that with more collaboration and knowledge sharing, collective understanding of the challenges of manufacturing vaccines at scale will improve, allowing governments and institutional purchasers who are investing in vaccine development programs and manufacturing capacity to better identify potential gaps or shortcomings. Such models may also provide a picture of when a vaccine is likely to be in our hands.






