Bifurcated needles could be the key to mpox vaccination — but what are they?
As experts work to contain the mpox outbreak, PATH investigates whether the tool that helped eradicate smallpox could expedite lifesaving immunization.
- 20 September 2024
- 4 min read
- by PATH
Since 2022, cases of mpox—the disease caused by monkeypox virus (MPXV)— have been surging in the Democratic Republic of the Congo. Cases are increasing in neighboring countries as well and have been detected in countries in Asia and Europe.
The current outbreak, fueled by a new strain that may be spreading more easily between humans, poses a serious global threat. Last month the World Health Organization (WHO) declared the current outbreak a public health emergency of international concern.
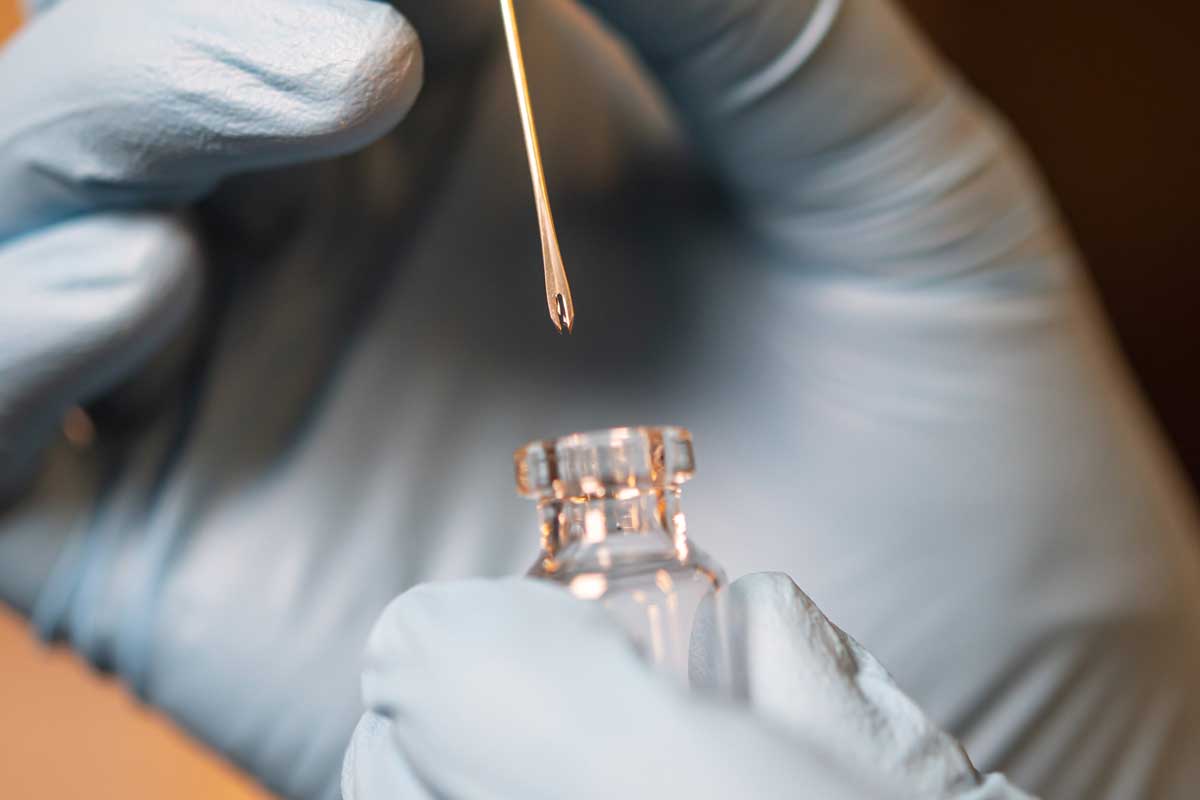
As public health experts work rapidly to contain the outbreak, PATH is investigating how a lesser-known tool—the bifurcated needle—could help expedite lifesaving vaccination campaigns. PATH’s Courtney Jarrahian, Global Program Leader, Medical Devices and Health Technologies, explains what it is and how it works.
What are bifurcated needles?
Many people may be familiar with vaccine injections administered with a needle and a syringe—this device can perform either an intradermal injection (into the skin), a subcutaneous injection (under the skin), or an intramuscular injection (deep into a muscle).
Two-prong, bifurcated needles administer a small droplet of vaccine held between the prongs by piercing only the top-most layer of the skin.
Bifurcated needles were widely used decades ago in WHO smallpox vaccine campaigns, as they were a low-cost and effective means of administering millions of smallpox vaccines. This worked because smallpox vaccines are unique in that only a tiny amount—less than 1 microliter (a typical intradermal injection is 100 microliters)—scratched onto the surface of the skin replicates and provides an effective immune response.
These immunization efforts ultimately led to the global eradication of smallpox.
How could these needles support mpox response?
Smallpox and monkeypox are in the same virus family, and smallpox vaccines provide cross-protection against MPXV as well. This means that stockpiles of smallpox vaccines could potentially be used to prevent and respond to mpox outbreaks.
However, once smallpox was eradicated, use of the vaccine was discontinued along with the bifurcated needles that made global immunization possible. Therefore, securing supply of the devices and providing training to health care workers is critical to successfully use this vaccine for mpox response.
The COVID-19 pandemic highlighted the importance of planning ahead to ensure vaccines as well as vaccine delivery devices are available in communities at the same time and in the same place. These logistics can be challenging: mpox vaccines and COVID-19 vaccines can require specialized devices.
For example, some COVID-19 vaccines have a different dose volume than typical vaccines—these required new sizes of immunization syringes to be rapidly approved, manufactured, and distributed globally.
In the case of mpox, the available vaccines each have different delivery methods: subcutaneous or intradermal injection with a needle and syringe, or skin administration with a bifurcated needle.
It’s critical to ensure the correct devices are supplied with vaccine doses. However, this is challenging because it’s still unclear which vaccine will be available for countries facing mpox outbreaks.
How is PATH partnering with stakeholders to address these challenges?
PATH is providing technical assistance to UNICEF to support device procurement for mpox vaccines. As part of this support, we are conducting testing of bifurcated needles in PATH’s Product Development and Engineering Laboratory to determine whether devices meet the required specifications for mpox vaccine delivery. Our engineers are conducting rapid testing to confirm bifurcated needles from the limited number of existing suppliers deliver the correct amount of vaccine.
However, there is much more work to do to prepare to immunize at-risk populations. This is one of the many massive gaps in mpox response at the moment.
Part of this preparation must include training health care workers. Most of the current generation of health care workers were born after the last case of naturally occurring smallpox was reported in 1977. For this reason, most aren’t familiar with bifurcated needles.
Planning for immunization logistics, training, needle safety, and community communication will be essential to facilitate successful immunization efforts.
What experience does PATH bring to this work?
PATH collaborated with global stakeholders during the COVID-19 pandemic to provide technical assistance and market analytics on immunization syringes. This experience is informing efforts to ensure delivery device supply for mpox immunization.
We also have a long history of working with the wide range of alternative devices that have been developed for intradermal delivery of vaccines, including evaluating technical function, safety, efficacy, usability, acceptability, health economics, and programmatic fit.
We are hopeful this expertise can help inform mpox vaccine research and rollout.