Credit: J&J. *Source: WHO factsheet.
Dr. Paul Stoffels, Chief Scientific Officer and Worldwide Chairman, Pharmaceuticals, Johnson & Johnson.
Ebola continues to cause suffering among patients, families and health care workers in West Africa. In response, the world’s scientific, pharmaceutical, regulatory, and public health communities have united in taking action to find new ways to collaborate and drive forward innovations to help prevent Ebola infection. These collaborative efforts are happening at record pace, and they offer a window into what is possible in the face of a global epidemic.
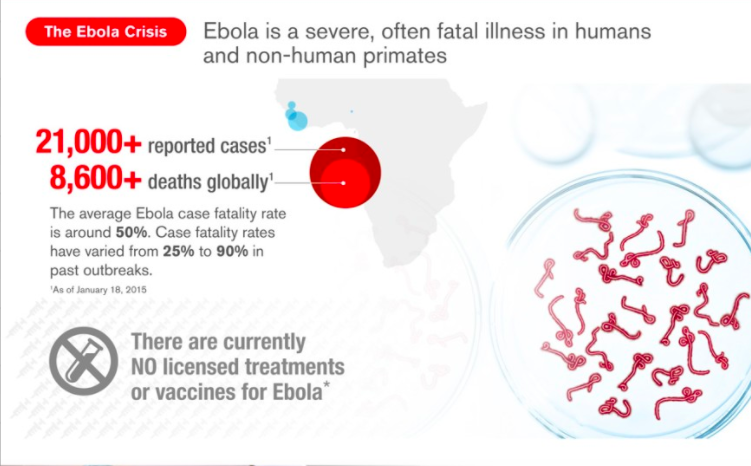
Speed matters. To date, almost 8,000 people have died from Ebola and more than 20,000 cases have been reported and the numbers continue to rise.
This sense of urgency has motivated our teams to work day and night in our labs and in close collaboration with partners such as the World Health Organization, U.S. Centers for Disease Control and Prevention, UK Medicines and Healthcare Products Regulatory Agency on finding a solution as fast as possible. Together we are working to accelerate the process of developing, producing and distributing a preventive Ebola vaccine regimen.
As a result of these collaborative efforts, today Johnson & Johnson announced the start of a Phase 1 first-in-human clinical trial of a preventive Ebola vaccine regimen in development at our Janssen Pharmaceutical Companies. The first-in-human study will evaluate the safety and tolerability of a prime-boost vaccine regimen, in which patients are first given a prime to the immune system, and then a boost intended to enhance the immune response over time. The immune response generated by the regimen will also be evaluated longer term.

Credit: J&J. Find more information here.
Testing of a new vaccine regimen is only one part of the equation. We need to ensure that if the vaccine is approved by appropriate health authorities, no time is wasted in making it available to the people at greatest risk. Because every day counts we are substantially accelerating the production of our vaccine regimen to ensure that if the vaccine is approved, it will be immediately available. To date, we have produced more than 400,000 regimens of the prime-boost vaccine for use in large-scale clinical trials by April.
All of us – across governments, health authorities, NGOs and industry – are dedicated to working together and pushing as hard and as quickly as we can to help fight Ebola’s spread. Through this unprecedented collaboration among the global health community, I’m hopeful we will meet our goal as fast as possible so that those at greatest risk may be protected for the long term.
Originally posted here. Dr. Paul Stoffels is Chief Scientific Officer, and Worldwide Chairman, Pharmaceuticals, Johnson & Johnson. In this role, he works with R&D leaders across Johnson & Johnson to set the enterprise-wide innovation agenda and is a member of the Johnson & Johnson Executive Committee. He began his career as a physician in Africa, focusing on HIV and tropical diseases research. Paul chairs the Johnson & Johnson R&D Management Committee and provides oversight to the Johnson & Johnson Development Corporation (JJDC) and the Johnson & Johnson innovation centers, with the goal of catalyzing innovative science and technology.