How improved yellow fever diagnostics are transforming management of the disease
Gavi has supported a major expansion in yellow fever diagnostic capacity in Africa over the past three years. The results show just how much improving diagnostics can have a cost-effective yet significant impact on immunisation programmes.
- 7 September 2021
- 7 min read
- by Gavi Staff

Yellow fever, a mosquito-borne viral hemorrhagic fever that can cause uncontrolled bleeding and death, clinically resembles many other diseases, meaning accurate diagnostic testing is essential for determining whether someone has yellow fever or another disease, such as Ebola. As well as affecting how that individual is treated, this diagnostic confirmation also feeds into yellow fever surveillance, helping to identify which areas do and do not need to be prioritised for vaccination, facilitating more efficient, equitable, and effective use of yellow fever vaccine.
In 2018, the Gavi Alliance and countries at high risk of yellow fever outbreaks in Africa faced a challenge in determining when and where to use vaccines to prevent yellow fever because of major gaps in diagnostic testing capacity.
If yellow fever outbreaks can be quickly identified and then contained through mass vaccination campaigns, the amount of death and disease from the outbreak and the cost of containing it will be smaller than for a larger outbreak that has had more time to expand.
For example, if a 2015–2016 yellow fever outbreak in Luanda, Angola had been stopped before it spread to the Democratic Republic of Congo, not only would there have been markedly fewer yellow fever cases and deaths, but a mass vaccination campaign that cost Gavi alone US$ 12 million would have been unnecessary. Conversely, diagnostic testing can rule out yellow fever as the cause of outbreaks of other diseases that look like yellow fever, avoiding unnecessary outbreak response vaccination campaigns.
WHY IS GAVI INVESTING IN DIAGNOSTICS?
Gavi places a high value on preventing yellow fever, allocating US$ 424 million for yellow fever vaccination during 2021–2025 in support of the global Eliminate Yellow Fever Epidemics (EYE) strategy. More efficient, equitable, and effective yellow fever vaccination can have very substantial benefits not only in terms of deaths prevented but also in terms of money saved.
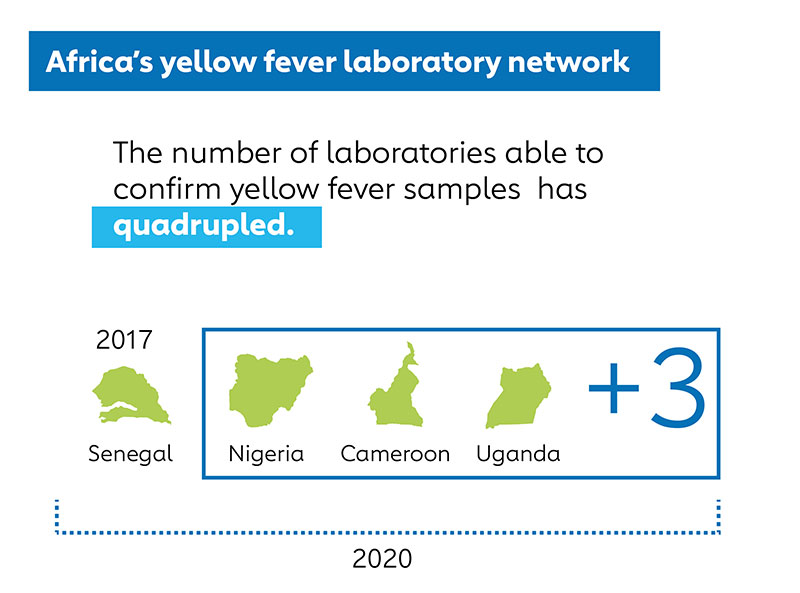
However, in 2018, the Gavi Alliance and countries at high risk of yellow fever outbreaks in Africa faced a challenge in determining when and where to use vaccines to prevent yellow fever because of major gaps in diagnostic testing capacity. Only one laboratory in Africa, the Institut Pasteur Dakar in Senegal, had the capacity to fully confirm if a suspected case of yellow fever really was yellow fever, so all other countries in Africa had to send samples internationally to determine if they might have a yellow fever outbreak.
A lack of validated commercial yellow fever test kits also meant that national public health laboratories had to pull together over a dozen different chemicals from various suppliers to conduct the first line yellow fever test - a laboratory test for IgM antibodies to yellow fever virus. These complicated logistics could cause supply stockouts, preventing laboratories from conducting even the first line tests to determine if a sample needed to be sent for confirmatory testing.
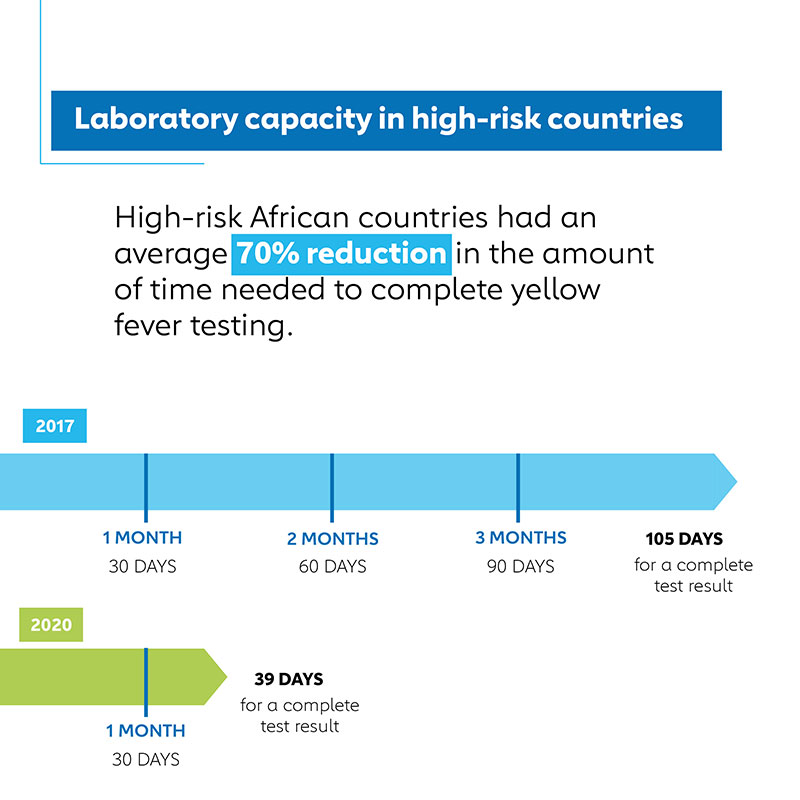
These factors, exacerbated by a frequent lack of funding for international sample shipments and periodic disruptions to international shipping routes, meant that for each sample from a suspected yellow fever case that quite possibly indicated a new or expanded yellow fever outbreak, it took on average three and a half months to conduct all the laboratory tests to determine if it really was yellow fever.
Have you read?
Even though such results might have been accurate, they clearly were often not timely enough to inform decisions on how to target yellow fever vaccination, particularly in response to possible yellow fever outbreaks.
HOW THE INVESTMENT WORKED
To address these gaps in diagnostic capacity, the Gavi Alliance took a multifaceted approach. It created a pooled procurement mechanism to buy yellow fever diagnostic test kits for Gavi-eligible African countries at high risk of yellow fever. UNICEF Supply Division engaged with diagnostic manufacturers and established new supply routes.
The WHO Eliminate Yellow Fever Epidemics (EYE) Laboratory Technical Working Group (LTWG) also developed specifications for what kinds of tests were worth using and organised evaluations of test kits to determine which ones could reliably provide accurate results. To reduce international shipping times, WHO worked with shipping companies to set up the EYE-OPS international sample transportation system with Gavi funding, and provided training to national laboratory logisticians on how to best navigate the complexities of international sample transportation.
To increase the number of laboratories able to confirm yellow fever in at least some of their own samples, WHO, the Centers for Disease Control and Prevention, and laboratories such as the Institute Pasteur Dakar, Uganda Virus Research Institute, Centre Pasteur Dakar, and the Robert Koch Institute provided technical assistance and training, including on newer polymerase chain reaction (PCR) testing which allows national laboratories to confirm yellow fever from some samples themselves, without referral to reference laboratories in other countries. All these efforts complemented work by national public health institutes, such as the Nigeria Centre for Disease Control and the Institut Pasteur of Cote d’Ivoire, to improve their own yellow fever diagnostic testing and surveillance.
THE RESULTS
As a result of these efforts, marked progress has been made in closing diagnostic testing gaps for yellow fever. This year the EYE LTWG validated a PCR test kit made by German company Altona Diagnostics for use in national public health laboratories in the WHO yellow fever laboratory network, and additional test kits and rapid diagnostic tests for yellow fever antibodies are under evaluation. Manufacturers have developed innovative tests for yellow fever NS1 protein - a non-structural protein produced by yellow fever virus - which could further facilitate testing in very resource-limited settings. Four African countries (Senegal, Cameroon, Uganda, and Nigeria) can now confirm at least some yellow fever samples, and that number is expected to increase as validated yellow fever PCR test kits become more widely used by trained laboratorians.
According to surveys of African countries at high risk for yellow fever epidemics eligible for Gavi support, the amount of time needed from arrival of samples at national laboratories to completion of testing dropped 70% from an average of 106 days in 2017 to 39 days in 2020 for samples that tested positive for yellow fever at a national laboratory. This progress is particularly noteworthy considering the disruptions to supply, shipping and disease surveillance systems caused by the COVID-19 pandemic.
While diagnostic capacity gaps remain, the progress to date provides a basis for further improvements, particularly given the relatively low cost of this program compared to the vaccination program it supports. Gavi has committed US$ 424 million during 2021–2025 to yellow fever vaccine procurement and distribution, primarily in Africa. In contrast, its efforts to address yellow fever diagnostic capacity are expected to cost US$ 8.1 million for the 2019–2022 period.
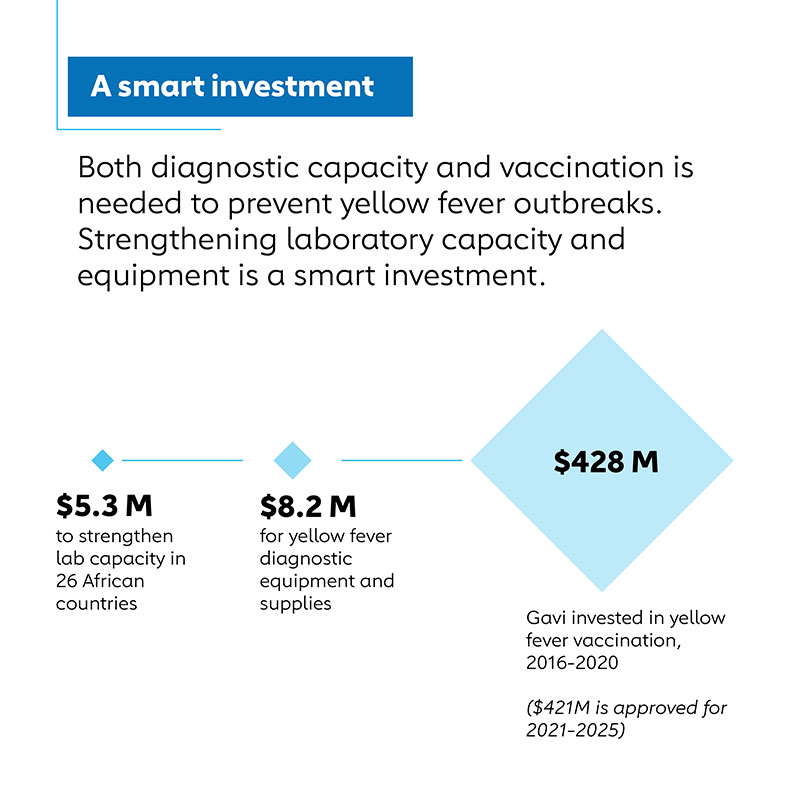
Even more importantly, the costs of yellow fever diagnostic tests to the countries that need them are relatively modest. For example, the cost of reagents and consumable supplies for the first line ELISA test for IgM antibodies to yellow fever virus supplied through UNICEF averages US$ 12,000 per year for the 21 African countries at high risk for yellow fever epidemics receiving Gavi diagnostic procurement support.
Already, the data from these diagnostic tests are being used to drive immunization program decisions. For instance, in the last three years they have prompted Uganda, which is at high risk for yellow fever epidemics, to introduce lifesaving yellow fever vaccine into routine immunization programmes. They have also indicated that at least three disease outbreaks suspected of being yellow fever in various countries were not in fact yellow fever, averting unnecessary and potentially costly yellow fever outbreak response campaigns.
Given the death and disruption that yellow fever can cause, diagnostic testing to improve the effectiveness, efficiency, and equity of yellow fever immunization programs is a bargain.








